Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells
- PMID: 31040708
- PMCID: PMC6459152
- DOI: 10.2147/IDR.S197531
Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells
Abstract
Background: The deep-seated infections caused by the Candida genus are associated with a high mortality rate, and Candida albicans is the most frequent species associated with these diseases. The fungal wall is composed of macromolecules not synthesized by the host, and therefore is a source of ligands recognized by innate immune cells.
Methods: We performed a comparative study analyzing the cell wall composition and organization of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris, along with their ability to stimulate cytokine production and phagocytosis by human innate immune cells.
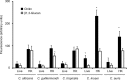
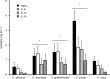
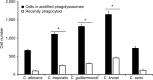
Results: We found that the wall of these species had the basic components already described in C. albicans, with most of the chitin and b1,3-glucan located underneath the mannan layer. However, the walls of C. krusei and C. auris were rich in chitin and the former had a lower content of mannans. C. guilliermondii contained changes in the mannan and the b1,3-glucan levels. These species were differentially phagocytosed by human macrophages and stimulated cytokine production in a dectin-1-dependent pathway. C. krusei showed the most significant changes in the tested parameters, whereas C. auris behaved like C. albicans.
Conclusion: Our results suggest that the cell wall and innate immune recognition of C. tropicalis, C. guilliermondii, C. krusei, and Candida auris is different from that reported for C. albicans.
Keywords: cell wall; cytokine production; host–fungus interplay; phagocytosis; protein glycosylation.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Pappas PG, Rex JH, Lee J, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37(5):634–643. - PubMed
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv113. - PubMed
-
- Lass-Flörl C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses. 2009;52(3):197–205. - PubMed
-
- Tan TY, Tan AL, Tee NWS, Ng LSY, Chee CWJ. The increased role of non-albicans species in candidaemia: results from a 3-year surveillance study. Mycoses. 2010;53(6):515–521. - PubMed
LinkOut - more resources
Full Text Sources

