Microbial-Based Double-Stranded RNA Production to Develop Cost-Effective RNA Interference Application for Insect Pest Management
- PMID: 31040730
- PMCID: PMC6482651
- DOI: 10.1177/1179543319840323
Microbial-Based Double-Stranded RNA Production to Develop Cost-Effective RNA Interference Application for Insect Pest Management
Abstract
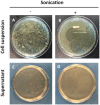
RNA interference (RNAi) is a convenient tool to identify and characterize biological functions in organisms. Recently, it has become an alternative to chemical insecticides as a biologically based control agent. This promising technology has the potential to avoid many problems associated with conventional chemical insecticides. In order for RNAi application to be practical for field use, a major hurdle is the development of a cost-effective system of double-stranded RNA (dsRNA) production for a large quantity of dsRNA. A handful of research reports has demonstrated microbial-based dsRNA production using L4440 vector and HT115 (DE3) Escherichia coli for application to vertebrate and invertebrate systems. However, the dsRNA yield, production efficiency, and biological purity from this in vitro system is still unclear. Thus, our study detailed biochemical and molecular tools for large-scale dsRNA production using the microbial system and investigated the production efficiency and yield of crude and purified dsRNAs. An unrelated insect gene, green fluorescent protein (GFP), and an insect neuropeptide gene, pyrokinin (PK) identified from Drosophila suzukii, were used to construct the recombinant L4440 to be expressed in the HT115 (DE3) cell. A considerable amount of dsRNA, 19.5 µg/mL of liquid culture, was isolated using ultrasonic disruption followed by phenol extraction. The sonication method was further evaluated to extract crude dsRNA without the additional phenol extraction and nuclease treatments and also to reduce potential bacterial viability. The results suggest that the ultrasonic method saved time and costs to isolate crude dsRNA directly from large volumes of cell culture without E coli contamination. We investigated whether the injection of PK dsRNA into flies resulted in increased adult mortality, but it was not statistically significant at 95% confidence level. In this study, the microbial-based dsRNA production has potential for applied RNAi technology to complement current insect pest management practices.
Keywords: RNAi; bacterial dsRNA production; pest control; spotted wing drosophila.
Conflict of interest statement
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S-JA, KD, YK, RRM, and M-YC have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Figures



References
-
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Mao YB, Cai WJ, Wang JW, et al. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol. 2007;25:1307–1313. - PubMed
-
- Baum JA, Bogaert T, Clinton W, et al. Control of coleopteran insect pests through RNA interference. Nat Biotechnol. 2007;25:1322–1326. - PubMed
-
- Zotti MJ, Smagghe G. RNAi technology for insect management and protection of beneficial insects from diseases: lessons, challenges and risk assessments. Neotrop Entomol. 2015;44:197–213. - PubMed
-
- Gundersen-Rindal DE, Adrianos SL, Allen ML, et al. Arthropod genomics research in the United States Department of Agriculture Agricultural Research Service: applications of RNA interference and CRISPR gene editing technologies in pest control. Trends Entomol. 2017;13:109–137.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

