Drosophila Nrf2/Keap1 Mediated Redox Signaling Supports Synaptic Function and Longevity and Impacts on Circadian Activity
- PMID: 31040766
- PMCID: PMC6476960
- DOI: 10.3389/fnmol.2019.00086
Drosophila Nrf2/Keap1 Mediated Redox Signaling Supports Synaptic Function and Longevity and Impacts on Circadian Activity
Abstract
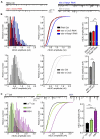
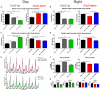
Many neurodegenerative conditions and age-related neuropathologies are associated with increased levels of reactive oxygen species (ROS). The cap "n" collar (CncC) family of transcription factors is one of the major cellular system that fights oxidative insults, becoming activated in response to oxidative stress. This transcription factor signaling is conserved from metazoans to human and has a major developmental and disease-associated relevance. An important mammalian member of the CncC family is nuclear factor erythroid 2-related factor 2 (Nrf2) which has been studied in numerous cellular systems and represents an important target for drug discovery in different diseases. CncC is negatively regulated by Kelch-like ECH associated protein 1 (Keap1) and this interaction provides the basis for a homeostatic control of cellular antioxidant defense. We have utilized the Drosophila model system to investigate the roles of CncC signaling on longevity, neuronal function and circadian rhythm. Furthermore, we assessed the effects of CncC function on larvae and adult flies following exposure to stress. Our data reveal that constitutive overexpression of CncC modifies synaptic mechanisms that positively impact on neuronal function, and suppression of CncC inhibitor, Keap1, shows beneficial phenotypes on synaptic function and longevity. Moreover, supplementation of antioxidants mimics the effects of augmenting CncC signaling. Under stress conditions, lack of CncC signaling worsens survival rates and neuronal function whilst silencing Keap1 protects against stress-induced neuronal decline. Interestingly, overexpression and RNAi-mediated downregulation of CncC have differential effects on sleep patterns possibly via interactions with redox-sensitive circadian cycles. Thus, our data illustrate the important regulatory potential of CncC signaling in neuronal function and synaptic release affecting multiple aspects within the nervous system.
Keywords: Drosophila neuromuscular junction; Keap1; Nrf2; longevity; redox signaling; sleep; synaptic release.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

