Structural Vaccinology for Viral Vaccine Design
- PMID: 31040832
- PMCID: PMC6476906
- DOI: 10.3389/fmicb.2019.00738
Structural Vaccinology for Viral Vaccine Design
Abstract
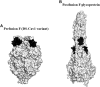
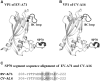
Although vaccines have proven pivotal against arrays of infectious viral diseases, there are still no effective vaccines against many viruses. New structural insights into the viral envelope, protein conformation, and antigenic epitopes can guide the design of novel vaccines against challenging viruses such as human immunodeficiency virus (HIV), hepatitis C virus, enterovirus A71, and dengue virus. Recent studies demonstrated that applications of this structural information can solve some of the vaccine conundrums. This review focuses on recent advances in structure-based vaccine design, or structural vaccinology, for novel and innovative viral vaccine design.
Keywords: epitope; protein structure; structural vaccinology; vaccine design; virus.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

