Investigational growth factors utilized in animal models of spinal fusion: Systematic review
- PMID: 31041160
- PMCID: PMC6475812
- DOI: 10.5312/wjo.v10.i4.176
Investigational growth factors utilized in animal models of spinal fusion: Systematic review
Abstract
Background: Over 400000 Americans annually undergo spinal fusion surgeries, yet up to 40% of these procedures result in pseudoarthrosis even with iliac crest autograft, the current "gold standard" treatment. Tissue engineering has the potential to solve this problem via the creation of bone grafts involving bone-promoting growth factors (e.g., bone morphogenetic protein 2). A broad assessment of experimental growth factors is important to inform future work and clinical potential in this area. To date, however, no study has systematically reviewed the investigational growth factors utilized in preclinical animal models of spinal fusion.
Aim: To review all published studies assessing investigational growth factors for spinal fusion in animal models and identify promising agents for translation.
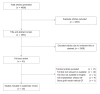
Methods: We conducted a systematic review of the literature using PubMed, Embase, Cochrane Library, and Web of Science databases with searches run on May 29th, 2018. The search query was designed to include all non-human, preclinical animal models of spinal fusion reported in the literature without a timespan limit. Extracted data for each model included surgical approach, level of fusion, animal species and breed, animal age and sex, and any other relevant characteristics. The dosages/sizes of all implant materials, spinal fusion rates, and follow-up time points were recorded. The data were analyzed and the results reported in tables and text. PRISMA guidelines were followed for this systematic review.
Results: Twenty-six articles were included in this study, comprising 14 experimental growth factors: AB204 (n = 1); angiopoietin 1 (n = 1); calcitonin (n = 3); erythropoietin (n = 1); basic fibroblast growth factor (n = 1); growth differentiation factor 5 (n = 4), combined insulin-like growth factor 1 + transforming growth factor beta (n = 4); insulin (n = 1); NELL-1 (n = 5); noggin (n = 1); P-15 (n = 1); peptide B2A (n = 2); and secreted phosphoprotein 24 (n = 1). The fusion rates of the current gold standard treatment (autologous iliac crest bone graft, ICBG) and the leading clinically used growth factor (BMP-2) ranged widely in the included studies, from 0-100% for ICBG and from 13%-100% for BMP-2. Among the identified growth factors, calcitonin, GDF-5, NELL-1, and P-15 resulted in fusion rates of 100% in some cases. In addition, six growth factors - AB204, angiopoietin 1, GDF-5, insulin, NELL-1, and peptide B2A - resulted in significantly enhanced fusion rates compared to ICBG, BMP-2, or other internal control in some studies. Large heterogeneity in animal species, fusion method, and experimental groups and time points was observed across the included studies, limiting the direct comparison of the growth factors identified herein.
Conclusion: Several promising investigational growth factors for spinal fusion have been identified herein; directly comparing the fusion efficacy and safety of these agents may inform clinical translation.
Keywords: Growth factor; Pseudoarthrosis; Spinal fusion; Systematic review.
Conflict of interest statement
Conflict-of-interest statement: Goodwin CR: Grants from NIH/NINDS K12 Physician Scientist Award, North Carolina Spine Society, and Burroughs Wellcome Funds; all outside the submitted work. Lo SF: Research support from Chordoma Foundation, Grant from AO Spine; all outside the submitted work. Sciubba DM: Consulting from Baxter, DePuy Synthes, Globus, K2M, Medical Device Business Services, Medtronic, NuVasive, and Stryker; Speaking/Teaching from Globus Medical, Medtronic, and DePuy Synthes; all outside the submitted work. Theodore N: Royalties from Globus Medical, Depuy Synthes; Stock in Globus Medical; Consulting from Globus Medical; Scientific Advisory Board from Globus Medical; Fellowship Support from AO North America; all outside the submitted work. Witham TF: Grants from Eli Lilly Company and the Gordon and Marilyn Macklin Foundation.
Figures
References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. - PMC - PubMed
-
- Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67–76. - PubMed
-
- Buchbinder R, van Tulder M, Öberg B, Costa LM, Woolf A, Schoene M, Croft P Lancet Low Back Pain Series Working Group. Low back pain: a call for action. Lancet. 2018;391:2384–2388. - PubMed
-
- Albert TJ, Pinto M, Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion. A functional and radiographic outcome study. Spine (Phila Pa 1976) 2000;25:123–9; discussion 130. - PubMed
-
- Cheh G, Bridwell KH, Lenke LG, Buchowski JM, Daubs MD, Kim Y, Baldus C. Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine (Phila Pa 1976) 2007;32:2253–2257. - PubMed
LinkOut - more resources
Full Text Sources