Clinical, genetic, and pathologic characterization of FKRP Mexican founder mutation c.1387A>G
- PMID: 31041397
- PMCID: PMC6454397
- DOI: 10.1212/NXG.0000000000000315
Clinical, genetic, and pathologic characterization of FKRP Mexican founder mutation c.1387A>G
Abstract
Objective: To characterize the clinical phenotype, genetic origin, and muscle pathology of patients with the FKRP c.1387A>G mutation.
Methods: Standardized clinical data were collected for all patients known to the authors with c.1387A>G mutations in FKRP. Muscle biopsies were reviewed and used for histopathology, immunostaining, Western blotting, and DNA extraction. Genetic analysis was performed on extracted DNA.
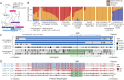
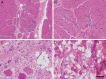
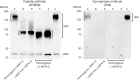
Results: We report the clinical phenotypes of 6 patients homozygous for the c.1387A>G mutation in FKRP. Onset of symptoms was <2 years, and 5 of the 6 patients never learned to walk. Brain MRIs were normal. Cognition was normal to mildly impaired. Microarray analysis of 5 homozygous FKRP c.1387A>G patients revealed a 500-kb region of shared homozygosity at 19q13.32, including FKRP. All 4 muscle biopsies available for review showed end-stage dystrophic pathology, near absence of glycosylated α-dystroglycan (α-DG) by immunofluorescence, and reduced molecular weight of α-DG compared with controls and patients with homozygous FKRP c.826C>A limb-girdle muscular dystrophy.
Conclusions: The clinical features and muscle pathology in these newly reported patients homozygous for FKRP c.1387A>G confirm that this mutation causes congenital muscular dystrophy. The clinical severity might be explained by the greater reduction in α-DG glycosylation compared with that seen with the c.826C>A mutation. The shared region of homozygosity at 19q13.32 indicates that FKRP c.1387A>G is a founder mutation with an estimated age of 60 generations (∼1,200-1,500 years).
Figures

References
-
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 1992;355:696–702. - PubMed
-
- Henry MD, Campbell KP. Dystroglycan inside and out. Curr Opin Cell Biol 1999;11:602–607. - PubMed
-
- Cohn RD. Dystroglycan: important player in skeletal muscle and beyond. Neuromuscul Disord 2005;15:207–217. - PubMed
-
- Kanagawa M, Kobayashi K, Tajiri M, et al. Identification of a post-translational modification with ribitol-phosphate and its defect in muscular dystrophy. Cell Rep 2016;14:2209–2223. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
