Photoacoustic elastography imaging: a review
- PMID: 31041859
- PMCID: PMC6990059
- DOI: 10.1117/1.JBO.24.4.040902
Photoacoustic elastography imaging: a review
Abstract
Elastography imaging is a promising tool-in both research and clinical settings-for diagnosis, staging, and therapeutic treatments of various life-threatening diseases (including brain tumors, breast cancers, prostate cancers, and Alzheimer's disease). Large variation in the physical (elastic) properties of tissue, from normal to diseased stages, enables highly sensitive characterization of pathophysiological states of the diseases. On the other hand, over the last decade or so, photoacoustic (PA) imaging-an imaging modality that combines the advantageous features of two separate imaging modalities, i.e., high spatial resolution and high contrast obtainable, respectively, from ultrasound- and optical-based modalities-has been emerging and widely studied. Recently, recovery of elastic properties of soft biological tissues-in addition to prior reported recovery of vital tissue physiological information (Hb, HbO2, SO, and total Hb), noninvasively and nondestructively, with unprecedented spatial resolution (μm) at penetration depth (cm)-has been reported. Studies demonstrating that combined recovery of mechanical tissue properties and physiological information-by a single (PA) imaging unit-pave a promising platform in clinical diagnosis and therapeutic treatments. We offer a comprehensive review of PA imaging technology, focusing on recent advances in relation to elastography. Our review draws out technological challenges pertaining to PA elastography (PAE) imaging, and viable approaches. Currently, PAE imaging is in the nurture stage of its development, where the technology is limited to qualitative study. The prevailing challenges (specifically, quantitative measurements) may be addressed in a similar way by which ultrasound elastography and optical coherence elastography were accredited for quantitative measurements.
Keywords: diagnosis; elastography; photoacoustic imaging; physiological activities; soft tissues; therapeutic treatments.
Figures



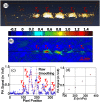

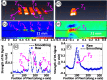
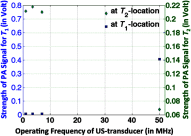
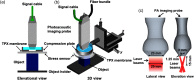
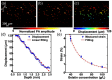
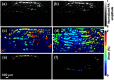
References
-
- Fung Y. C., Biomechanics: Mechanical Properties of Living Tissue, Springer-Verlag, New York: (1993).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

