A gain-of-function sodium channel β2-subunit mutation in painful diabetic neuropathy
- PMID: 31041876
- PMCID: PMC6510061
- DOI: 10.1177/1744806919849802
A gain-of-function sodium channel β2-subunit mutation in painful diabetic neuropathy
Abstract
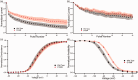
Diabetes mellitus is a global challenge with many diverse health sequelae, of which diabetic peripheral neuropathy is one of the most common. A substantial number of patients with diabetic peripheral neuropathy develop chronic pain, but the genetic and epigenetic factors that predispose diabetic peripheral neuropathy patients to develop neuropathic pain are poorly understood. Recent targeted genetic studies have identified mutations in α-subunits of voltage-gated sodium channels (Navs) in patients with painful diabetic peripheral neuropathy. Mutations in proteins that regulate trafficking or functional properties of Navs could expand the spectrum of patients with Nav-related peripheral neuropathies. The auxiliary sodium channel β-subunits (β1-4) have been reported to increase current density, alter inactivation kinetics, and modulate subcellular localization of Nav. Mutations in β-subunits have been associated with several diseases, including epilepsy, cancer, and diseases of the cardiac conducting system. However, mutations in β-subunits have never been shown previously to contribute to neuropathic pain. We report here a patient with painful diabetic peripheral neuropathy and negative genetic screening for mutations in SCN9A, SCN10A, and SCN11A-genes encoding sodium channel α-subunit that have been previously linked to the development of neuropathic pain. Genetic analysis revealed an aspartic acid to asparagine mutation, D109N, in the β2-subunit. Functional analysis using current-clamp revealed that the β2-D109N rendered dorsal root ganglion neurons hyperexcitable, especially in response to repetitive stimulation. Underlying the hyperexcitability induced by the β2-subunit mutation, as evidenced by voltage-clamp analysis, we found a depolarizing shift in the voltage dependence of Nav1.7 fast inactivation and reduced use-dependent inhibition of the Nav1.7 channel.
Keywords: Diabetic neuropathy; neuropathic pain; sodium channel beta-subunits; voltage-gated sodium channels.
Figures



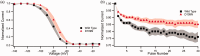
References
-
- Carracher AM, Marathe PH, Close KL. International diabetes federation 2017. J Diabetes 2018; 10: 353–356. - PubMed
-
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993; 36: 150–154. - PubMed
-
- Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O’Brien PC, Melton LJ III, Service FJ. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993; 43: 817–824. - PubMed
-
- Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014; 371: 1131–1141. - PubMed
-
- Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. . Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem 1994; 269: 9889–9897. - PubMed

