Prostacyclin for pulmonary arterial hypertension
- PMID: 31042010
- PMCID: PMC6492481
- DOI: 10.1002/14651858.CD012785.pub2
Prostacyclin for pulmonary arterial hypertension
Abstract
Background: Pulmonary arterial hypertension (PAH) is characterised by pulmonary vascular changes, leads to elevated pulmonary artery pressures, dyspnoea, a reduction in exercise tolerance, right heart failure, and ultimately death.Prostacyclin analogue drugs mimic endogenous prostacyclin which leads to vasodilation, inhibition of platelet aggregation, and reversal of vascular remodelling. Prostacyclin's short half-life theoretically enhances selectivity for the pulmonary vascular bed by direct (via central venous catheter) administration. Initial continuous infusion prostacyclins were efficacious, but use of intravenous access increases the risk of adverse events. Newer and safer subcutaneous, oral and inhaled preparations are now available, though possibly less potent.Selexipag is an oral selective prostacyclin receptor (IP receptor) agonist that works similarly to prostacyclin, potentially more stable, with less complex administration and titration.
Objectives: To determine the efficacy and safety of prostacyclin, prostacyclin analogues or prostacyclin receptor agonists for PAH in adults and children.
Search methods: We performed searches on CENTRAL, MEDLINE, and Embase up to 16 September 2018. We handsearched review articles, clinical trial registries, and reference lists of retrieved articles.
Selection criteria: We included any randomised controlled trials (RCTs) which compared prostacyclin, prostacyclin analogues or prostacyclin receptor agonists to control (placebo, any other treatment or usual care) for at least six weeks.
Data collection and analysis: We used standard methods specified by Cochrane. Primary outcomes included change in World Health Organization (WHO) functional class, six-minute walk distance (6MWD), and mortality.
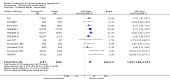
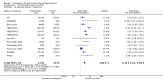
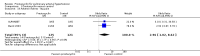
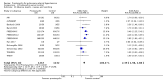
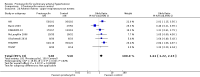
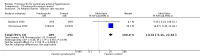
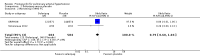
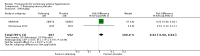
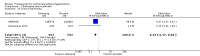
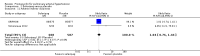
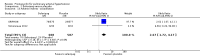
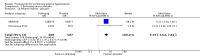
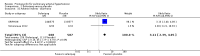
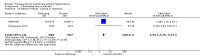
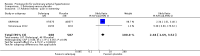
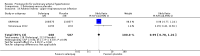
Main results: Seventeen trials with 3765 mostly adult participants were included; median trial duration was 12 weeks. Fifteen trials used prostacyclin analogues: intravenous (N = 4); subcutaneous (N = 1); oral (N = 5); inhaled (N = 5); two used oral prostacyclin receptor agonists. Three intravenous and two inhaled trials were open-label.Participants using prostacyclin had 2.39 times greater odds of improving by at least one WHO functional class (95% confidence interval (CI) 1.72 to 3.32; 24 per 100 (95% CI 18.5 to 30.4) with prostacyclin compared to 12 per 100 with control; 8 trials, 1066 participants; moderate-certainty evidence). Improvement occurred with intravenous (odds ratio (OR) 14.96, 95% CI 4.76 to 47.04), and inhaled (OR 2.94, 95% CI 1.53 to 5.66), but not with oral preparations. Participants using prostacyclin increased their 6MWD by 19.50 metres (95% CI 14.82 to 24.19; 13 trials, 2283 participants; low-certainty evidence), which was clinically significant with intravenous (mean difference (MD) 91.76 metres; 95% CI 58.97 to 124.55), but not with non-intravenous preparations (subcutaneous: MD 16.00 metres, 95% CI 7.38 to 24.62; oral: MD 14.76 metres, 95% CI 7.81 to 21.70; inhaled: MD 26.97 metres, 95% CI 17.21 to 36.73). Mortality was reduced in the intravenous (OR 0.29, 95% CI 0.12 to 0.69; risk of death 6 per 100 (95% CI 2.38 to 12.31) with prostacyclin compared to 17 per 100 with control; 4 trials, 255 participants), but not in the non-intravenous studies (OR 0.82, 95% CI 0.48 to 1.40; risk of death 21 per 1000 (95% CI 12.00 to 34.20) with prostacyclin compared to 25 per 1000 with control; moderate-certainty evidence; 12 trials, 2299 participants). We reduced the certainty of evidence due to few studies per subgroup and use of open-label trials.Prostacyclins improved cardiopulmonary haemodynamics (reduction in mean pulmonary artery pressure by 3.60 mmHg (95% CI -4.73 to -2.48); pulmonary vascular resistance by 2.81 WU (95% CI -3.80 to -1.82); right atrial pressure by 1.90 mmHg (95% CI -2.58 to -1.22), and increase in cardiac index by 0.31 L/min/m2 (95% CI 0.23 to 0.38); low-certainty evidence), improved dyspnoea (low-certainty evidence, and improved quality of life (moderate-certainty evidence), when compared to control. When only subcutaneous/inhaled trials were included the effect was still significant, but the magnitude was smaller. There was no difference across oral trials.Adverse events were increased in all prostacyclin preparations, including vasodilation (OR 5.03, 95% CI 3.84 to 6.58), headache (OR 3.16, 95% CI 2.62 to 3.80), jaw pain (OR 5.25, 95% CI 3.96 to 6.98), diarrhoea (OR 2.81, 95% CI 2.29 to 3.46), nausea/vomiting (OR 2.39, 95% CI 1.98 to 2.88), myalgias (OR 2.75, 95% CI 1.65 to 4.58), upper respiratory tract events (OR 1.61, 95% CI 1.22 to 2.13), extremity pain (OR 3.36, 95% CI 2.32 to 4.85), and infusion site reactions (OR 14.41, 95% CI 9.16 to 22.66). In the intravenous trials, there was a 12%-25% risk of serious non-fatal events including sepsis, haemorrhage, pneumothorax and pulmonary embolism.Two trials (1199 participants) compared oral selexipag to placebo; no trials compared selexipag with prostacyclin. There was a small 12.62 metre improvement in 6MWD (95% CI 1.90 to 23.34; high-certainty evidence), and weak evidence for haemodynamics. The effect was uncertain for WHO functional class. The risk of death with selexipag was five per 100 compared to three per 100 with placebo, though the CI crossed zero so the true effect is uncertain (risk difference (RD) 0.02 (95% CI -0.00 to 0.04). There was less clinical worsening with selexipag (OR 0.47, 95% CI 0.37 to 0.60), though more side effects, including vasodilation (OR 2.67, 95% CI 1.72 to 4.17), headache (OR 3.91, 95% CI 3.07 to 4.98), jaw pain (OR 5.33, 95% CI 3.64 to 7.81), diarrhoea (OR 3.11, 95% CI 2.39 to 4.05), nausea/vomiting (OR 2.92, 95% CI 2.29 to 3.73), pain in the extremities (OR 2.44, 95% CI 1.69 to 3.52), and myalgias (OR 3.05, 95% CI 2.02 to 4.58).
Authors' conclusions: This review demonstrates clinical and statistical benefit for intravenous prostacyclin (compared to control) with improved functional class, 6MWD, mortality, symptoms scores, and cardiopulmonary haemodynamics, but at a cost of adverse events. This may be due to a true effect, or may be overestimated due to the inclusion of small, short or open-label studies. There was a statistical and small clinical benefit in function and haemodynamics for inhaled prostacyclin, but the effect is uncertain for mortality. The effect of oral prostacyclins are less certain. Selexipag demonstrated less clinical worsening without discernable impact on survival, increased adverse events; and the effect on other outcomes is less certain. Real-world registry data may provide further information about clinical effect.
Conflict of interest statement
HB: has previously been awarded a travel scholarship that was determined by an independent abstract committee, funded by GSK. GSK had no involvement in the determination of recipients. The Cochrane funding arbiter was consulted and agreed it did not represent a conflict of interest as GSK had no role in the allocation of the travel scholarship.
HLY: none known
TF: none known
AB: none known
MH: has relationships with drug companies in the field of pulmonary hypertension including Actelion, Arena, Bayer, Eiger, GSK, and United Therapeutics. In addition to being investigator in trials involving these companies, relationships include consultancy service and membership of scientific advisory boards. MH has relationships with drug companies in the field of severe asthma outside the submitted work. These companies are Astrazeneca, GSK, MSD, Novartis, Roche/Genentech, Sanofi/Regereron, and Teva. In addition to being investigator in trials involving these companies, relationships include consultancy service and membership of scientific advisory boards.
TW: is a paid member of scientific advisory boards for Actelion Australia, GSK Australia and Bayer Australia. He has received travel support, consultancy payments and research/education grants from Actelion Australia.
Figures
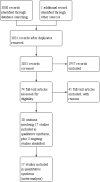
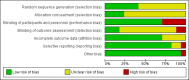




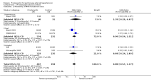






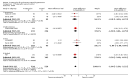













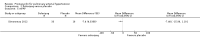
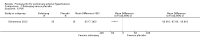
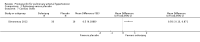
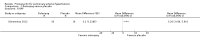



Update of
- doi: 10.1002/14651858.CD012785
References
References to studies included in this review
AIR {published data only}
-
- Ghofrani A. Positive outcome of the Aerosolized Iloprost Randomized study in primary and non‐primary pulmonary hypertension (AIR study). Journal fur Anasthesie und Intensivbehandlung 2002;9(2):41.
-
- Olschewski H, Galie N, Higenbottam T, Naeije R, Rubin LJ, Simonneau G, et al. Positive outcome of the aerosolized iloprost randomised trial for treatment of severe pulmonary hypertension (AIR study). American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A411.
-
- Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. New England Journal of Medicine 2002;347(5):322‐9. - PubMed
ALPHABET {published data only}
-
- Galie N, Humbert M, Vachiery JL, Vizza CD, Kneussl M, Manes A, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double‐blind, placebo‐controlled trial. Journal of the American College of Cardiology 2002;39(9):1496‐502. - PubMed
-
- Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Effects of beraprost sodium, an oral prostacyclin analogue in patients with pulmonary arterial hypertension: a randomised, double‐blind, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;165(6):800‐4. - PubMed
Badesch 2000 {published data only}
-
- Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Annals of Internal Medicine 2000;132(6):425‐34. - PubMed
Barst 1996 {published data only}
-
- Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. New England Journal of Medicine 1996; Vol. 334:296‐301. - PubMed
-
- Hinderliter AL, Willis PW 4th, Barst RJ, Rich S, Rubin LJ, Badesch DB. Effects of long‐term infusion of prostacyclin (epoprostenol) on echocardiographic measures of right ventricular structure and function in primary pulmonary hypertension. Primary Pulmonary Hypertension Study Group. Circulation 1997;95(6):1479‐86. - PubMed
Barst 2003 {published data only}
-
- Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, et al. Beraprost Study Group. Beraprost therapy for pulmonary arterial hypertension. Journal of the American College of Cardiology 2003;41(12):2119‐25. - PubMed
FREEDOM‐C {published data only}
-
- Ruggiero RM, Bartolome S, Chin KM, Torres F. Combination therapy for pulmonary arterial hypertension with oral treprostinil: long‐term follow up [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183:A5906.
-
- Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM‐C study): a randomized controlled trial. Chest 2012;142:1383‐90. - PubMed
FREEDOM‐C2 {published data only}
-
- Tapson V, Jing ZC, Xu K, Pan L, Feldman J, Kiely D, et al. Freedom‐C2: efficacy and safety of oral treprostinil diethanolamine in combination with an era and/or a PDE5 inhibitor in patients with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2012; Vol. 185:A2493.
-
- Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM‐C2 study): a randomized controlled trial. Chest 2013;144:952‐8. - PubMed
FREEDOM‐M {published data only}
-
- Jing ZC, Parikh K, Pulido T, Jerjes‐Sanchez C, White RJ, Allen R, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013;127:624‐33. - PubMed
-
- Rubin L, Parikh K, Pulido T, Jerjes‐Sanchez C, Allen R, White J, et al. FREEDOM‐M: efficacy and safety of oral treprostinil diethanolamine as monotherapy in patients with pulmonary arterial hypertension [Abstract]. Chest 2011;140(4):1044A.
GRIPHON {published data only}
-
- Channick R, Chin K, Scala L, Frey A, Preiss R, Gaine S, et al. Individualized dosing of selexipag based on tolerability in the GRIPHON study shows consistent efficacy and safety in patients with pulmonary arterial hypertension (PAH). Chest 2015;184:961A.
-
- Galie N, Channick R, Chin K, Frey A, Gaine S, Ghofrani A, et al. Effect of selexipag on morbidity/mortality in pulmonary arterial hypertension: Results of the griphon study. Journal of Heart and Lung Transplantation 2015;34:S163.
-
- Lang I, Gaine S, Galie N, Ghofrani HA, Brun FO, McLaughlin V, et al. Effect of selexipag on long‐term outcomes in patients with pulmonary arterial hypertension (PAH) receiving one, two or no PAH therapies at baseline: Results from the GRIPHON study. European Heart Journal 2015;36:381‐2.
-
- McLaughlin VV, Channick R, Chin K, Frey A, Gaine S, Ghofrani A, et al. Effect of selexipag on morbidity/mortality in pulmonary arterial hypertension: Results of the GRIPHON study. Journal of the American College of Cardiology 2015;65:A1538.
-
- Sitbon O, Channick R, Chin K, Frey A, Galie N, Ghofrani HA, et al. Effect of selexipag on longterm outcomes in key subgroups of patients with pulmonary arterial hypertension (PAH): GRIPHON study results. European Respiratory Journal 2015;46(Suppl 59):OA4995.
Han 2017 {published data only}
-
- Han X, Zhang Y, Dong L, Fang L, Chai Y, Niu M, et al. Treatment of pulmonary arterial hypertension using initial combination therapy of bosentan and iloprost. Respiratory Care 2017;62(4):489‐96. - PubMed
McLaughlin 2006 {published data only}
-
- McLaughlin VV, Oudiz R, Frost A, Tapson V, Murali S, Channick R, et al. A randomised, double‐blind, placebo‐controlled study of iloprost inhalation as add‐on therapy to bosentan in pulmonary arterial hypertensions (PAH). Chest 2005;128(4):160S.
-
- McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2006;174:1257‐63. - PubMed
Olschewski 2010 {published data only}
-
- Olschewski H, Hoeper MM, Behr J, Ewert R, Meyer A, Borst MM, et al. Long‐term therapy with inhaled iloprost in patients with pulmonary hypertension. Respiratory Medicine 2010;104(5):731‐40. - PubMed
Rubin 1990 {published data only}
-
- Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Annals of Internal Medicine 1990;112(7):485‐91. - PubMed
Simonneau 2002 {published data only}
-
- Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double‐blind, randomized, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;165(6):800‐4. - PubMed
Simonneau 2012 {published data only}
-
- Simonneau G, Lang I, Torbicki A, Hoeper MM, Delcroix M, Karlocai K, et al. Efficacy, safety and tolerability of ACT‐293987, a novel oral, non‐prostanoid, prostaglandin I2 (IP) receptor agonist: results from a phase IIa study in pulmonary arterial hypertension (PAH). American Journal of Respiratory and Critical Care Medicine 2010;181:A2515.
-
- Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlocai K, Galie N, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. European Respiratory Journal 2012;40(4):874‐80. - PubMed
-
- Torbicki A, Lang I, Hoeper M, Delcroix M, Karlocai K, Galie N, et al. A new drug class for Pulmonary Arterial Hypertension (PAH): results from a phase II study of ACT 293987, an oral IP receptor agonist. European Society of Cardiology Congress; 2010 28 Aug ‐ 1 Sep; Stockholm. 2010:22.
TRIUMPH {published data only}
-
- McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. Journal of the American College of Cardiology 2010;4(55):1915‐22. - PubMed
TRUST {published data only}
-
- Hiremath J, Thanikachalam S, Parikh K, Shanmugasundaram S, Bangera S, Shapiro L, et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo‐controlled trial. Journal of Heart and Lung Transplantation 2010;29:137‐49. - PubMed
References to studies excluded from this review
Bruderer 2014 {published data only}
-
- Bruderer S, Hurst N, Kaufmann P, Dingemanse J. Multiple‐dose up‐titration study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of selexipag, an orally available selective prostacyclin receptor agonist, in healthy subjects. Pharmacology 2014;94(3‐4):148‐56. - PubMed
Herald 2006 {published data only}
-
- Herald N, Laliberte K. Treprostinil for the treatment of pulmonary arterial hypertension in patients with New York Heart Association class II‐IV symptoms. Pharmacotherapy 2006;26(8):1203‐4. - PubMed
Higenbottam 1996 {published data only}
-
- Higenbottam T. Intravenous epoprostenol for primary pulmonary hypertension. New England Journal of Medicine 1996;334(22):1477‐8. - PubMed
Higenbottam 1998 {published data only}
Jing 2013 {published data only}
-
- Jing ZC, Zhao QH, Jiang X, Peng FH, Wu WH, He J, et al. Addition of beraprost to existing sildenafil therapy in patients with pulmonary arterial hypertension: a randomized, open‐label study (Best study). American Journal of Respiratory and Critical Care Medicine 2013;187:A6066.
Klings 2000 {published data only}
-
- Klings ES, Farber HW. Epoprostenol for pulmonary hypertension in scleroderma. Annals of Internal Medicine 2000;133(2):158. - PubMed
Kumar 2013 {published data only}
-
- Kumar P, Arneson C, Laliberte K, Nelsen AC. Dose‐response relationship of oral treprostinil diolamine (TRE) in patients with pulmonary arterial hypertension (PAH). American Thoracic Society International Conference; 2013 May 17‐22; Philadelphia. 2013; Vol. 187:A3271.
Kunieda 2013 {published data only}
-
- Kunieda T. Clinical trial of long‐acting beraprost sodium in Japanese patients with pulmonary arterial hypertension. Therapeutic Research 2013;34(9):1203‐6.
Leuchte 2003 {published data only}
-
- Leuchte HH, Baumgartner RA, Behr J. Treatment of severe pulmonary hypertension with inhaled iloprost. Annals of Internal Medicine 2003;139(4):306. - PubMed
Matthes 2001 {published data only}
-
- Matthes J, Mathen F, Herzig S, Wassermann K. Therapy of severe pulmonary hypertension with prostacyclin and iloprost. Deutsche Medizinische Wochenschrift (1946) 2001;126(21):631‐7. - PubMed
NCT00709098 2008 {unpublished data only}
-
- NCT00709098. Safety study extension of iloprost power 15 in pulmonary arterial hypertension (PROWESS 15 Ext). clinicaltrials.gov/ct2/show/NCT00709098 (first received 3 July 2008).
NCT00709956 2008 {unpublished data only}
-
- NCT00709956. Iloprost power 15 in pulmonary arterial hypertension (PROWESS 15). clinicaltrials.gov/ct2/show/NCT00709956 (first received 3 July 2008).
NCT00760916 2008 {unpublished data only}
-
- NCT00760916. FREEDOM DR: oral treprostinil in combination with an endothelin receptor antagonist (ERA) and/or a phosphodiesterase‐5 (PDE‐5) inhibitor or as monotherapy for the treatment of pulmonary arterial hypertension (PAH). clinicaltrials.gov/ct2/show/NCT00760916 (first received 26 September 2008).
NCT00993408 2009 {unpublished data only}
-
- NCT00993408. Study of ACT‐293987 (NS‐304) in pulmonary arterial hypertension (PAH). clinicaltrials.gov/ct2/show/NCT00993408 (first received 12 October 2009).
NCT01094067 2010 {unpublished data only}
-
- NCT01094067. Tezosentan in patients with pulmonary arterial hypertension. clinicaltrials.gov/ct2/show/NCT01094067 (first received 26 March 2010).
NCT01105091 2010 {unpublished data only}
-
- NCT01105091. Epoprostenol for injection in pulmonary arterial hypertension (EPITOME‐1). clinicaltrials.gov/ct2/show/NCT01105091 (first received 16 April 2010).
NCT01302444 2011 {unpublished data only}
-
- NCT01302444. Treprostinil combined with tadalafil for pulmonary hypertension (T2). clinicaltrials.gov/ct2/show/NCT01302444 (first received 24 February 2011).
NCT01393795 2011 {unpublished data only}
-
- NCT01393795. Qutenza®‐Remodulin® in pulmonary arterial hypertension patients. clinicaltrials.gov/ct2/show/NCT01393795 (first received 13 July 2011).
NCT01557647 2012 {unpublished data only}
-
- NCT01557647. Safety and efficacy of inhaled treprostinil in patients with PAH. clinicaltrials.gov/ct2/show/NCT01557647 (first received 19 March 2012).
NCT01598441 2012 {unpublished data only}
-
- NCT01598441. Multicenter study of iloprost inhaled in pulmonary hypertension after repair of congenital heart diseases (CHD). clinicaltrials.gov/ct2/show/NCT01598441 (first received 15 May 2012).
NCT02032836 2014 {unpublished data only}
-
- NCT02032836. Comparative PK PD Study in PAH patients (Fox vs. I‐Neb). clinicaltrials.gov/ct2/show/NCT02032836 (first received 10 January 2014).
NCT02482402 2014 {unpublished data only}
-
- NCT02482402. Iloprost for bridging to heart transplantation in PH (BRIDGE). clinicaltrials.gov/ct2/show/NCT02482402 (first received 26 June 2015).
NCT02893995 2016 {unpublished data only}
-
- NCT02893995. Safety, tolerability, pharmacokinetics and efficacy of two different rates of subcutaneous remodulin® dose titration in pulmonary arterial hypertension. clinicaltrials.gov/ct2/show/NCT02893995 (first received 9 September 2016).
NCT02999906 2016 {unpublished data only}
-
- NCT02999906. Study to compare triple therapy (oral treprostinil, ambrisentan, and tadalafil) with dual therapy (ambrisentan, tadalafil, and placebo) in subjects with pulmonary arterial hypertension. clinicaltrials.gov/ct2/show/NCT02999906 (first received 21 December 2016).
Olschewski 1998 {published data only}
-
- Olschewski H, Walmrath D, Schermuly R, Ghofrani HA, Grimminger F, Seeger W. Prostacyclin and iloprost in aerosol form in severe pulmonary hypertension. Pneumologie 1998;52(1):3‐7. - PubMed
Pepke‐Zaba 2000 {published data only}
-
- Pepke‐Zaba J, Parameshwar J, Eiskjaer H, Sharples L, Mainwood A, McNeil K. A comparison of three month's treatment with nebulised iloprost (Ilo) and continuous intravenous prostacyclin (PG1²) infusion for severe pulmonary hypertension (PH). European Respiratory Journal 2000;16(Suppl 31):395s.
-
- Pepke‐Zaba J, Parameshwar J, Eiskjaer H, Sharples L, Mainwood A, McNeil K. A comparison of three month's treatment with nebulised iloprost (Ilo) and continuous intravenous prostacyclin (PGI2) infusion for severe pulmonary hypertension (PH). American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A214.
Preston 2015 {published data only}
-
- Preston I, Hill N, Ghofrani HA, Hoeper M, Langleben D, Vizza CD, et al. Riociguat in combination with prostacyclin analogs for the treatment of pulmonary arterial hypertension (PAH): a subgroup analysis of the PATENT studies. Chest 2015;148:4.
Robbins 2000 {published data only}
-
- Robbins I, Gaine S, Schilz R, Tapson V, Rubin L, Loyd J. Epoprostenol for treatment of pulmonary hypertension in patients with systemic lupus erythematosus. Chest 2000;117(1):14‐8. - PubMed
Rubenfire 2007 {published data only}
-
- Rubenfire M, McLaughlin VV, Allen RP, Elliott G, Park MH, Wade M, et al. Transition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trial. [Erratum appears in Chest. 2007 Nov;132(5): 1721]. Chest 2007;132(3):757‐63. - PubMed
Rubin 2005 {published data only}
-
- Rubin L. Epoprostenol and nesiritide in pulmonary hypertension. Chest 2005;127(5):1870. - PubMed
Saba 2001 {published data only}
-
- Saba T, Peacock AJ. Long‐term treatment of pulmonary hypertension with aerosolized iloprost. European Respiratory Journal 2001;18(1):247. - PubMed
Saggar 2013 {published data only}
-
- Saggar R, White RJ, Rischard F, Mathier M, Elwing J, Allen RP, et al. Effect of oral treprostinil on hemodynamics during exercise in patients with pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine 2013;187:A3281.
Shah 2010 {published data only}
-
- Shah AM, Siddiqui F, Preston IR, Brennan J, Roberts K, Howard W, et al. Comparison of acute hemodynamic effects of inhaled nitric oxide (iNo) and inhaled epoprostenol (iEPO) in patients with pulmonary arterial hypertension (PAH) [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181:A3382.
Voswinckel 2006 {published data only}
-
- Voswinckel R, Enke B, Reichenberger F, Kohstall M, Kreckel A, Krick S, et al. Favorable effects of inhaled treprostinil in severe pulmonary hypertension: results from randomized controlled pilot studies. Journal of the American College of Cardiology 2006;48(8):1672‐81. - PubMed
Voswinckel 2006a {published data only}
-
- Voswinckel R, Ghofrani HA, Grimminger F, Seeger W, Olschewski H. Inhaled treprostinil [corrected] for treatment of chronic pulmonary arterial hypertension. [Erratum appears in Ann Intern Med. 2006 Jun 6;144(11):863]. Annals of Internal Medicine 2006;144(2):149‐50. - PubMed
Wade 2007 {published data only}
-
- Wade M. Intravenous treprostinil in pulmonary arterial hypertension: a controlled trial in India. Chest 2007;132(4):635b‐6. - PubMed
White 2013 {published data only}
-
- White RJ, Torres F, Allen R, Jerjes C, Pulido T, Yehle D, et al. Pharmacokinetics of oral treprostinil sustained release tablets during chronic administration to patients with pulmonary arterial hypertension. Journal of Cardiovascular Pharmacology 2013;61(6):474‐81. - PubMed
White 2013a {published data only}
-
- White RJ, Jing Z‐C, Parikh K, Bartolome S, Allen RP, Xu K‐F, et al. An open‐label extension trial of oral treprostinil in subjects with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2013;187:A3270.
Wilkens 2001 {published data only}
-
- Wilkens H, Groeschel‐Guth A, Koening J, Forestier N, Boehm M, Sybrecht GW. Inhaled iloprost plus oral sildenafil in patients with pulmonary hypertension. European Respiratory Journal 2001;18(Suppl 33):324s. - PubMed
Wilkens 2001a {published data only}
-
- Wilkens H, Guth A, Konig J, Forestier N, Cremers B, Hennen B, et al. Effect of inhaled Iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation 2001;104(11):1218‐22. - PubMed
Zamanian 2013 {published data only}
-
- Zamanian RT, Scott V, Marlin C, Gomberg‐Maitland M, Preston IR, Cuttica MJ. CONFRONT. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A3298.
References to ongoing studies
NCT 2013 {unpublished data only}
-
- NCT01908699. Beraprost‐314d added‐on to Tyvaso® (BEAT). clinicaltrials.gov/ct2/show/NCT01908699 (first received 26 July 2013).
NCT 2015 {published data only}
-
- NCT02558231. The efficacy and safety of initial triple versus initial dual oral combination therapy in patients with newly diagnosed pulmonary arterial hypertension. clinicaltrials.gov/ct2/show/NCT02558231 (first received 23 September 2015).
TRACE 2018 {published data only}
-
- Preston I, Frantz R, Hemnes A, Rosenkranz S, Skaara H, Pfister T, et al. Rationale and design of trace: a double‐blind, placebo‐controlled phase 4 study in pulmonary arterial hypertension to assess the effect of selexipag on physical activity, patient‐reported symptoms and their impact, in daily life. American Journal of Respiratory and Critical Care Medicine 2018;197:A5680.
Additional references
Badesch 2009
-
- Badesch DB. Diagnosis and assessment of pulmonary arterial hypertension. Journal of the American College of Cardiology 2009;54(Suppl 1):S55‐66. - PubMed
Cenedese 2006
-
- Cenedese E, Speich R, Dorschner L, Ulrich S, Maggiorini M, Jenni R, et al. Measurement of quality of life in pulmonary hypertension and its significance. European Respiratory Journal 2006;28(4):808‐15. - PubMed
Christman 1992
-
- Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. New England Journal of Medicine 1992;327(2):70‐5. - PubMed
D’Alonzo 1991
-
- D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Annals of Internal Medicine 1991;115:343–9. - PubMed
Enderby 2014
-
- Enderby CY, Soukup M, Al Omari M, Zeiger T, Burger C. Transition from intravenous or subcutaneous prostacyclin therapy to inhaled treprostinil inpatients with pulmonary arterial hypertension: a retrospective case series. Journal of Clinical Pharmacy and Therapeutics 2014;39(5):496‐500. - PubMed
Galiè 2016
-
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Revista Espanola de Cardiologia 2016;69(2):177. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 29 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 1989
-
- Guyatt GH, Nogradi S, Halcrow S, et al. Development and testing of a new measure of health status for clinical trials in heart failure. Journal of General Internal Medicine 1989;4:101‐7. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Humbert 2006
-
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. American Journal of Respiratory and Critical Care Medicine 2006;173(9):1023‐30. - PubMed
Humbert 2015
Kallen 2008
-
- Kallen AJ, Lederman E, Balaji A, Trevino I, Petersen EE, Shoulson R, et al. Bloodstream infections in patients given treatment with intravenous prostanoids. Infection Control and Hospital Epidemiology 2008;29(4):342–9. - PubMed
Khair 2016
Ling 2012
-
- Ling Y, Johnson MK, Kiely DG. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. American Journal of Respiratory and Critical Care Medicine 2012;186(8):790‐6. - PubMed
McKenna 2006
-
- McKenna S, Doughty N, Meads DM, Doward LC, Pepke‐Zaba J. The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR): a measure of health‐related quality of life and quality of life for patients with pulmonary hypertension. Quality of Life Research 2006;15:13‐15. - PubMed
McLaughlin 2002
-
- McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002;106:1477–82. - PubMed
Melian 2002
-
- Melian EB, Goa KL. Beraprost: a review of its pharmacology and therapeutic efficacy in the treatment of peripheral arterial disease and pulmonary arterial hypertension. Drugs 2002;62(1):107‐33. - PubMed
Mitchell 2014
Moher 2009
Noel 2017
-
- Noel ZR, Kido K, Macaulay TE. Selexipag for the treatment of pulmonary arterial hypertension. American Journal of Health‐System Pharmacy 2017;74(15):1135‐41. - PubMed
Peacock 2007
-
- Peacock AJ, Murphy NF, McMurray JJ. An epidemiological study of pulmonary arterial hypertension. European Respiratory Journal 2007;30(1):104‐9. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Simonneau 2013
-
- Simonneau G. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25):D34‐41. - PubMed
Sitbon 2002
-
- Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, et al. Long‐term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. Journal of the American College of Cardiology 2002;40:780–8. - PubMed
Sitbon 2012
-
- Sitbon O, Delcroix M, Bergot E, Boonstra A, Escribano, Subias P, et al. EPITOME‐2, An open‐label study evaluating a new formulation of epoprostenol sodium in pulmonary arterial hypertension patients switched from Flolan. American Journal of Respiratory and Critical Care Medicine 2012;185:A2500.
Sitbon 2016
Tapson 2006
-
- Tapson VF, McLaughlin VV, Gomberg‐Maitland M, Widlitz AC, Krichman AD, Laliberte KJ, et al. Delivery of intravenous treprostinil at low infusion rates using a miniaturized infusion pump in patients with pulmonary arterial hypertension. Journal of Vascular Access 2006;7(3):112‐7. - PubMed
Thenappan 2007
-
- Thenappan T, Shah SJ, Rich S, Gomberg‐Maitland M. A USA‐based registry for pulmonary arterial hypertension: 1982–2006. European Respiratory Journal 2007;30(6):1103–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

