Diaphragm contractile weakness due to reduced mechanical loading: role of titin
- PMID: 31042425
- PMCID: PMC6732419
- DOI: 10.1152/ajpcell.00509.2018
Diaphragm contractile weakness due to reduced mechanical loading: role of titin
Abstract
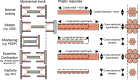
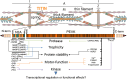
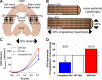
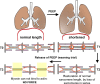
The diaphragm, the main muscle of inspiration, is constantly subjected to mechanical loading. Only during controlled mechanical ventilation, as occurs during thoracic surgery and in the intensive care unit, is mechanical loading of the diaphragm arrested. Animal studies indicate that the diaphragm is highly sensitive to unloading, causing rapid muscle fiber atrophy and contractile weakness; unloading-induced diaphragm atrophy and contractile weakness have been suggested to contribute to the difficulties in weaning patients from ventilator support. The molecular triggers that initiate the rapid unloading atrophy of the diaphragm are not well understood, although proteolytic pathways and oxidative signaling have been shown to be involved. Mechanical stress is known to play an important role in the maintenance of muscle mass. Within the muscle's sarcomere, titin is considered to play an important role in the stress-response machinery. Titin is a giant protein that acts as a mechanosensor regulating muscle protein expression in a sarcomere strain-dependent fashion. Thus titin is an attractive candidate for sensing the sudden mechanical arrest of the diaphragm when patients are mechanically ventilated, leading to changes in muscle protein expression. Here, we provide a novel perspective on how titin and its biomechanical sensing and signaling might be involved in the development of mechanical unloading-induced diaphragm weakness.
Keywords: diaphragm; loading; mechanical ventilation; titin.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. doi:10.1126/science.1065874. - DOI - PubMed
-
- Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol 306: 717–726, 2001. doi:10.1006/jmbi.2001.4448. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

