Selective EGLN Inhibition Enables Ablative Radiotherapy and Improves Survival in Unresectable Pancreatic Cancer
- PMID: 31043430
- PMCID: PMC6666414
- DOI: 10.1158/0008-5472.CAN-18-1785
Selective EGLN Inhibition Enables Ablative Radiotherapy and Improves Survival in Unresectable Pancreatic Cancer
Abstract
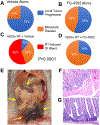
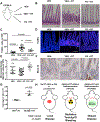
When pancreatic cancer cannot be removed surgically, patients frequently experience morbidity and death from progression of their primary tumor. Radiation therapy (RT) cannot yet substitute for an operation because radiation causes fatal bleeding and ulceration of the nearby stomach and intestines before achieving tumor control. There are no FDA-approved medications that prevent or reduce radiation-induced gastrointestinal injury. Here, we overcome this fundamental problem of anatomy and biology with the use of the oral EGLN inhibitor FG-4592, which selectively protects the intestinal tract from radiation toxicity without protecting tumors. A total of 70 KPC mice with autochthonous pancreatic tumors received oral FG-4592 or vehicle control ± ablative RT to a cumulative 75 Gy administered in 15 daily fractions to a limited tumor field. Although ablative RT reduced complications from local tumor progression, fatal gastrointestinal bleeding was observed in 56% of mice that received high-dose RT with vehicle control. However, radiation-induced bleeding was completely ameliorated in mice that received high-dose RT with FG-4592 (0% bleeding, P < 0.0001 compared with vehicle). Furthermore, FG-4592 reduced epithelial apoptosis by half (P = 0.002) and increased intestinal microvessel density by 80% compared with vehicle controls. EGLN inhibition did not stimulate cancer growth, as treatment with FG-4592 alone, or overexpression of HIF2 within KPC tumors independently improved survival. Thus, we provide a proof of concept for the selective protection of the intestinal tract by the EGLN inhibition to enable ablative doses of cytotoxic therapy in unresectable pancreatic cancer by reducing untoward morbidity and death from radiation-induced gastrointestinal bleeding. SIGNIFICANCE: Selective protection of the intestinal tract by EGLN inhibition enables potentially definitive doses of radiation therapy. This might allow radiation to be a surgical surrogate for unresectable pancreatic cancer.Graphical Abstract: http://cancerres.aacrjournals.org/content/canres/79/9/2327/F1.large.jpg.
©2019 American Association for Cancer Research.
Conflict of interest statement
Figures

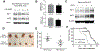

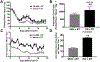
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21 - PubMed
-
- Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, et al. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2654–68 - PubMed
-
- Kelly P, Das P, Pinnix CC, Beddar S, Briere T, Pham M, et al. Duodenal toxicity after fractionated chemoradiation for unresectable pancreatic cancer. International journal of radiation oncology, biology, physics 2013;85:e143–9 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

