Antibiotics Enhance Prevention and Eradication Efficacy of Cathodic-Voltage-Controlled Electrical Stimulation against Titanium-Associated Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa Biofilms
- PMID: 31043516
- PMCID: PMC6495338
- DOI: 10.1128/mSphere.00178-19
Antibiotics Enhance Prevention and Eradication Efficacy of Cathodic-Voltage-Controlled Electrical Stimulation against Titanium-Associated Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa Biofilms
Abstract
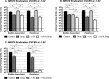
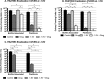
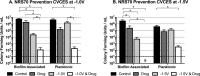
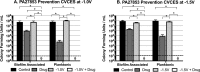
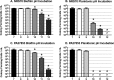
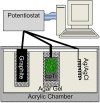
Periprosthetic joint infection (PJI) develops clinically, even with antibiotic treatment, and methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa are predominant causes of these infections. Due to biofilm formation, antibiotic treatment for patients with PJI can perpetuate resistance, further complicating the use of noninvasive treatments. This study evaluated cathodic-voltage-controlled electrical stimulation (CVCES) of titanium, in combination with a clinically relevant antibiotic, to synergistically prevent MRSA and P. aeruginosa PJIs by inhibiting bacterial adherence or as a treatment for eradicating established biofilms. CVCES of -1.0 V, -1.5 V, or -1.8 V (versus Ag/AgCl), with or without vancomycin for MRSA or gentamicin for P. aeruginosa, was applied to sterile titanium incubated with cultures to evaluate prevention of attachment or eradication of preestablished biofilms. Treatments were 24 h long and included open-circuit potential controls, antibiotic alone, CVCES, and CVCES plus antibiotic. Biofilm-associated and planktonic CFU were enumerated. In general, CVCES at -1.8 V alone or with antibiotic completely eradicated biofilm-associated CFU for both strains, and these parameters were also highly effective against planktonic bacteria, resulting in a >6-log reduction in MRSA and no detectable planktonic P. aeruginosa All CFU were reduced ∼3 to 5 logs from controls for prevention CVCES plus antibiotics at -1.0 V and -1.5 V against MRSA. Remarkably, there were no detectable P. aeruginosa CFU following prevention CVCES at -1.0 V or -1.5 V with gentamicin. Our results suggest that CVCES in combination with antibiotics may be an effective approach for prevention and treatment of PJI.IMPORTANCE Periprosthetic joint infections (PJIs) develop clinically in the presence of antibiotic therapies and are responsible for increased patient morbidity and rising health care costs. Many of these infections involve bacterial biofilm formation on orthopedic hardware, and it has been well established that these biofilms are refractory to most antibiotic treatments. Recent studies have focused on novel methods to prevent and eradicate infection. Cathodic-voltage-controlled electrical stimulation (CVCES) has previously been shown to be effective as a method for prevention and eradication of Gram-positive and Gram-negative infections. The present study revealed that the utility of CVCES for prevention and eradication of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa is enhanced in the presence of clinically relevant antibiotics. The synergistic effects of CVCES and antibiotics are effective in a magnitude-dependent manner. The results of this study indicate a promising alternative method to current PJI mitigation techniques.
Keywords: bacteria; biofilms; orthopedic infection; prevention; treatment.
Copyright © 2019 Canty et al.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
