Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis
- PMID: 31043573
- PMCID: PMC7106486
- DOI: 10.1126/scitranslmed.aat8329
Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis
Abstract
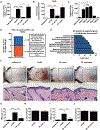
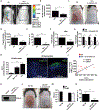
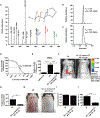
Colonization of the skin by Staphylococcus aureus is associated with exacerbation of atopic dermatitis (AD), but any direct mechanism through which dysbiosis of the skin microbiome may influence the development of AD is unknown. Here, we show that proteases and phenol-soluble modulin α (PSMα) secreted by S. aureus lead to endogenous epidermal proteolysis and skin barrier damage that promoted inflammation in mice. We further show that clinical isolates of different coagulase-negative staphylococci (CoNS) species residing on normal skin produced autoinducing peptides that inhibited the S. aureus agr system, in turn decreasing PSMα expression. These autoinducing peptides from skin microbiome CoNS species potently suppressed PSMα expression in S. aureus isolates from subjects with AD without inhibiting S. aureus growth. Metagenomic analysis of the AD skin microbiome revealed that the increase in the relative abundance of S. aureus in patients with active AD correlated with a lower CoNS autoinducing peptides to S. aureus ratio, thus overcoming the peptides' capacity to inhibit the S. aureus agr system. Characterization of a S. hominis clinical isolate identified an autoinducing peptide (SYNVCGGYF) as a highly potent inhibitor of S. aureus agr activity, capable of preventing S. aureus-mediated epithelial damage and inflammation on murine skin. Together, these findings show how members of the normal human skin microbiome can contribute to epithelial barrier homeostasis by using quorum sensing to inhibit S. aureus toxin production.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

References
-
- Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM, The burden of atopic dermatitis in US adults: Health care resource utilization data from the 2013 National Health and Wellness Survey. J. Am. Acad. Dermatol 78, 54–61.e1 (2018). - PubMed
-
- Narla S, Hsu DY, Thyssen JP, Silverberg JI, Inpatient financial burden of atopic dermatitis in the United States. J. Invest. Dermatol 137, 1461–1467 (2017). - PubMed
-
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O’Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH, Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet 38, 441–446 (2006). - PubMed
-
- De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoshida T, Boguniewicz M, Hata T, Schneider LC, Hanifin JM, Gallo RL, Novak N, Weidinger S, Beaty TH, Leung DY, Barnes KC, Beck LA, Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol 127, 773–786.e1-7 (2011). - PMC - PubMed
-
- Kato A, Fukai K, Oiso N, Hosomi N, Murakami T, Ishii M, Association of SPINK5 gene polymorphisms with atopic dermatitis in the Japanese population. British J. Dermatol 148, 665–669 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

