Aβ and tau prion-like activities decline with longevity in the Alzheimer's disease human brain
- PMID: 31043574
- PMCID: PMC6640844
- DOI: 10.1126/scitranslmed.aat8462
Aβ and tau prion-like activities decline with longevity in the Alzheimer's disease human brain
Abstract
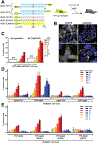
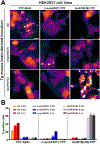
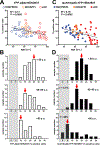
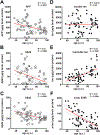
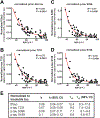
The hallmarks of Alzheimer's disease (AD) are the accumulation of Aβ plaques and neurofibrillary tangles composed of hyperphosphorylated tau. We developed sensitive cellular assays using human embryonic kidney-293T cells to quantify intracellular self-propagating conformers of Aβ in brain samples from patients with AD or other neurodegenerative diseases. Postmortem brain tissue from patients with AD had measurable amounts of pathological Aβ conformers. Individuals over 80 years of age had the lowest amounts of prion-like Aβ and phosphorylated tau. Unexpectedly, the longevity-dependent decrease in self-propagating tau conformers occurred in spite of increasing amounts of total insoluble tau. When corrected for the abundance of insoluble tau, the ability of postmortem AD brain homogenates to induce misfolded tau in the cellular assays showed an exponential decrease with longevity, with a half-life of about one decade over the age range of 37 to 99 years. Thus, our findings demonstrate an inverse correlation between longevity in patients with AD and the abundance of pathological tau conformers. Our cellular assays can be applied to patient selection for clinical studies and the development of new drugs and diagnostics for AD.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

References
-
- Alzheimer A, Über eigenartige Krankheitsfälle des späteren Alters. Zentralbl. Gesamte Neurol. Psychiatr 4, 356–385 (1911).
-
- Glenner GG, Wong CW, Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun 120, 885–890 (1984). - PubMed
-
- Brion JP, Couck AM, Passareiro E, Flament-Durand J, Neurofibrillary tangles of Alzheimer’s disease: an immunohistochemical study. J. Submicrosc. Cytol 17, 89–96 (1985). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG023501/AG/NIA NIH HHS/United States
- P01 AG002132/AG/NIA NIH HHS/United States
- R37 AG031220/AG/NIA NIH HHS/United States
- RF1 AG061874/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- MRC_/Medical Research Council/United Kingdom
- P50 NS062684/NS/NINDS NIH HHS/United States
- U01 AG006781/AG/NIA NIH HHS/United States
- R35 GM122603/GM/NIGMS NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

