Prognostic role of ARID1A negative expression in gastric cancer
- PMID: 31043675
- PMCID: PMC6494900
- DOI: 10.1038/s41598-019-43293-5
Prognostic role of ARID1A negative expression in gastric cancer
Abstract
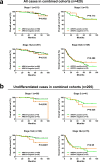
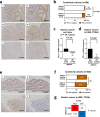
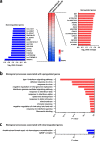
AT-rich interactive domain 1A (ARID1A) functions as a tumor suppressor and several therapeutic targets in ARID1A-mutated cancers are under development. Here, we investigated the prognostic value of ARID1A for gastric cancer and its association with expression of PD-L1 and p53. ARID1A expression was examined by immunohistochemistry and negative expression of ARID1A was detected in 39 (19.5%) of 200 cases in a test cohort and in 40 (18.2%) of 220 cases in a validation cohort. Negative expression of ARID1A was associated with worse overall survival in undifferentiated cases, particularly early-stage cases. Negative expression of ARID1A was detected in 11 (50%) of 22 PD-L1-positive cases and in 68 (17.1%) of 398 PD-L1-negative cases in a combined cohort. Negative expression of ARID1A was detected in 45 (22%) of 205 p53-positive cases and in 34 (15.8%) of 215 p53-negative cases in a combined cohort. In addition, expression of EZH2, a potential synthetic lethal target in ARID1A-mutated tumors, was detected in 79 ARID1A-negative cases. An ARID1A-knockdown gastric cancer cell line was subjected to microarray analysis, but no actionable targets or pathways were identified. The present results indicate that ARID1A may serve as an early-stage prognostic biomarker for undifferentiated gastric cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

