Arabidopsis FLL2 promotes liquid-liquid phase separation of polyadenylation complexes
- PMID: 31043738
- PMCID: PMC6625965
- DOI: 10.1038/s41586-019-1165-8
Arabidopsis FLL2 promotes liquid-liquid phase separation of polyadenylation complexes
Abstract
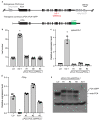
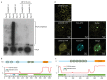
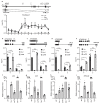
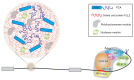
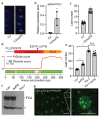
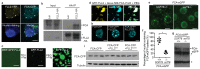
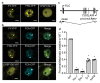
An important component of cellular biochemistry is the concentration of proteins and nucleic acids in non-membranous compartments1,2. These biomolecular condensates are formed from processes that include liquid-liquid phase separation. The multivalent interactions necessary for liquid-liquid phase separation have been extensively studied in vitro1,3. However, the regulation of this process in vivo is poorly understood. Here we identify an in vivo regulator of liquid-liquid phase separation through a genetic screen targeting factors required for Arabidopsis RNA-binding protein FCA function. FCA contains prion-like domains that phase-separate in vitro, and exhibits behaviour in vivo that is consistent with phase separation. The mutant screen identified a functional requirement for FLL2, a coiled-coil protein, in the formation of FCA nuclear bodies. FCA reduces transcriptional read-through by promoting proximal polyadenylation at many sites in the Arabidopsis genome3,4. FLL2 was required to promote this proximal polyadenylation, but not the binding of FCA to target RNA. Ectopic expression of FLL2 increased the size and number of FCA nuclear bodies. Crosslinking with formaldehyde captured in vivo interactions between FLL2, FCA and the polymerase and nuclease modules of the RNA 3'-end processing machinery. These 3' RNA-processing components colocalized with FCA in the nuclear bodies in vivo, which indicates that FCA nuclear bodies compartmentalize 3'-end processing factors to enhance polyadenylation at specific sites. Our findings show that coiled-coil proteins can promote liquid-liquid phase separation, which expands our understanding of the principles that govern the in vivo dynamics of liquid-like bodies.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/J/000PR9788/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- ERC_/European Research Council/International
- BBS/E/J/000PR9773/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_U105192713/MRC_/Medical Research Council/United Kingdom
- 210654/Z/18/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

