Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors
- PMID: 31043758
- PMCID: PMC6522365
- DOI: 10.1038/s41588-019-0403-1
Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors
Abstract
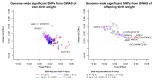
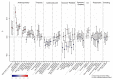
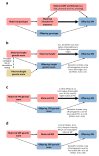
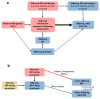
Birth weight variation is influenced by fetal and maternal genetic and non-genetic factors, and has been reproducibly associated with future cardio-metabolic health outcomes. In expanded genome-wide association analyses of own birth weight (n = 321,223) and offspring birth weight (n = 230,069 mothers), we identified 190 independent association signals (129 of which are novel). We used structural equation modeling to decompose the contributions of direct fetal and indirect maternal genetic effects, then applied Mendelian randomization to illuminate causal pathways. For example, both indirect maternal and direct fetal genetic effects drive the observational relationship between lower birth weight and higher later blood pressure: maternal blood pressure-raising alleles reduce offspring birth weight, but only direct fetal effects of these alleles, once inherited, increase later offspring blood pressure. Using maternal birth weight-lowering genotypes to proxy for an adverse intrauterine environment provided no evidence that it causally raises offspring blood pressure, indicating that the inverse birth weight-blood pressure association is attributable to genetic effects, and not to intrauterine programming.
Conflict of interest statement
A.A.V. is an employee of AstraZeneca. S.F.A.G. has received support from GSK for research that is not related to the study presented in this paper. D.A.L. has received support from Medtronic LTD and Roche Diagnostics for biomarker research that is not related to the study presented in this paper. M.I.M. serves on advisory panels for Pfizer, NovoNordisk, Zoe Global; has received honoraria from Merck, Pfizer, NovoNordisk and Eli Lilly; has stock options in Zoe Global; has received research funding from Abbvie, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, NovoNordisk, Pfizer, Roche, Sanofi Aventis, Servier, Takeda.
Figures
References
-
- Barker DJ, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. - PubMed
-
- Ben-Shlomo Y, Smith GD. Deprivation in infancy or in adult life: which is more important for mortality risk? Lancet. 1991;337:530–534. - PubMed
Publication types
MeSH terms
Grants and funding
- MC_UU_00007/10/MRC_/Medical Research Council/United Kingdom
- R24 ES028507/ES/NIEHS NIH HHS/United States
- R21 ES028226/ES/NIEHS NIH HHS/United States
- 202802/WT_/Wellcome Trust/United Kingdom
- MC_UU_12013/4/MRC_/Medical Research Council/United Kingdom
- 205915/WT_/Wellcome Trust/United Kingdom
- P30 ES010126/ES/NIEHS NIH HHS/United States
- MC_PC_19009/MRC_/Medical Research Council/United Kingdom
- MC_UU_00011/1/MRC_/Medical Research Council/United Kingdom
- MR/J012165/1/MRC_/Medical Research Council/United Kingdom
- MR/M005070/1/MRC_/Medical Research Council/United Kingdom
- 088806/WT_/Wellcome Trust/United Kingdom
- 104150/WT_/Wellcome Trust/United Kingdom
- G1001357/MRC_/Medical Research Council/United Kingdom
- MC_UU_12015/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12015/2/MRC_/Medical Research Council/United Kingdom
- MC_PC_15018/MRC_/Medical Research Council/United Kingdom
- P30 ES023515/ES/NIEHS NIH HHS/United States
- MC_UP_A620_1017/MRC_/Medical Research Council/United Kingdom
- R01 ES022223/ES/NIEHS NIH HHS/United States
- MC_UU_12013/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00011/6/MRC_/Medical Research Council/United Kingdom
- 212259/WT_/Wellcome Trust/United Kingdom
- R01 DK103246/DK/NIDDK NIH HHS/United States
- 098395/WT_/Wellcome Trust/United Kingdom
- R01 ES029212/ES/NIEHS NIH HHS/United States
- 17/0005594/DUK_/Diabetes UK/United Kingdom
- 208806/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- 323195/ERC_/European Research Council/International
- P30 ES019776/ES/NIEHS NIH HHS/United States
- MC_UU_00011/5/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/4/MRC_/Medical Research Council/United Kingdom
- 098381/WT_/Wellcome Trust/United Kingdom
- 202802/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- G0601261/MRC_/Medical Research Council/United Kingdom
- G9815508/MRC_/Medical Research Council/United Kingdom
- MC_UU_12013/2/MRC_/Medical Research Council/United Kingdom

