Heterotopic Ossification: A Comprehensive Review
- PMID: 31044187
- PMCID: PMC6478587
- DOI: 10.1002/jbm4.10172
Heterotopic Ossification: A Comprehensive Review
Abstract
Heterotopic ossification (HO) is a diverse pathologic process, defined as the formation of extraskeletal bone in muscle and soft tissues. HO can be conceptualized as a tissue repair process gone awry and is a common complication of trauma and surgery. This comprehensive review seeks to synthesize the clinical, pathoetiologic, and basic biologic features of HO, including nongenetic and genetic forms. First, the clinical features, radiographic appearance, histopathologic diagnosis, and current methods of treatment are discussed. Next, current concepts regarding the mechanistic bases for HO are discussed, including the putative cell types responsible for HO formation, the inflammatory milieu and other prerequisite "niche" factors for HO initiation and propagation, and currently available animal models for the study of HO of this common and potentially devastating condition. © 2019 The Authors. JBMR Plus published by Wiley Periodicals, Inc. on behalf of American Society for Bone and Mineral Research.
Keywords: ECTOPIC BONE; FIBRODYSPLASIA OSSIFICANS PROGRESSIVA; HETEROTOPIC BONE; MYOSITIS OSSIFICANS.
Figures
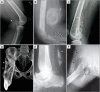
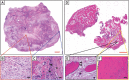
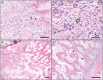

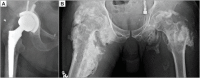
References
-
- Dorfman HD, Czerniak B. Bone tumors: Maryland Heights, MO: Mosby; 1998.
-
- Ackerman LV. Extra‐osseous localized non‐neoplastic bone and cartilage formation (so‐called myositis ossificans): clinical and pathological confusion with malignant neoplasms. J Bone Joint Surg Am. 1958;40‐A(2):279–98. - PubMed
-
- de Silva MV, Reid R. Myositis ossificans and fibroosseous pseudotumor of digits: a clinicopathological review of 64 cases with emphasis on diagnostic pitfalls. Int J Surg Pathol. 2003;11 (3):187–95. - PubMed
-
- Nuovo MA, Norman A, Chumas J, Ackerman LV. Myositis ossificans with atypical clinical, radiographic, or pathologic findings: a review of 23 cases. Skeletal Radiol. 1992;21 (2):87–101. - PubMed
-
- Rosenberg AE. Pseudosarcomas of soft tissue. Arch Pathol Lab Med. 2008;132(4):579–86. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
