Deletion of pancreatic β-cell adenosine kinase improves glucose homeostasis in young mice and ameliorates streptozotocin-induced hyperglycaemia
- PMID: 31044530
- PMCID: PMC6584724
- DOI: 10.1111/jcmm.14216
Deletion of pancreatic β-cell adenosine kinase improves glucose homeostasis in young mice and ameliorates streptozotocin-induced hyperglycaemia
Abstract
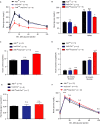
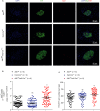
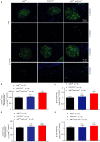
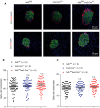
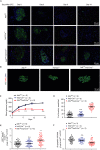
Severe reduction in the β-cell number (collectively known as the β-cell mass) contributes to the development of both type 1 and type 2 diabetes. Recent pharmacological studies have suggested that increased pancreatic β-cell proliferation could be due to specific inhibition of adenosine kinase (ADK). However, genetic evidence for the function of pancreatic β-cell ADK under physiological conditions or in a pathological context is still lacking. In this study, we crossed mice carrying LoxP-flanked Adk gene with Ins2-Cre mice to acquire pancreatic β -cell ADK deficiency (Ins2-Cre± Adkfl/fl ) mice. Our results revealed that Ins2-Cre+/- Adkfl/fl mice showed improved glucose metabolism and β-cell mass in younger mice, but showed normal activity in adult mice. Moreover, Ins2-Cre± Adkfl/fl mice were more resistant to streptozotocin (STZ) induced hyperglycaemia and pancreatic β-cell damage in adult mice. In conclusion, we found that ADK negatively regulates β-cell replication in young mice as well as under pathological conditions, such as STZ induced pancreatic β-cell damage. Our study provided genetic evidence that specific inhibition of pancreatic β-cell ADK has potential for anti-diabetic therapy.
Keywords: adenosine kinase; diabetes; insulin; replication; β cell.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
No potential conflicts of interest regarding this work were reported.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

