Differential transcriptome and development of human peripheral plasma cell subsets
- PMID: 31045577
- PMCID: PMC6538338
- DOI: 10.1172/jci.insight.126732
Differential transcriptome and development of human peripheral plasma cell subsets
Abstract
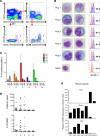
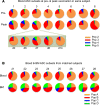
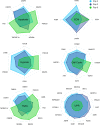
Human antibody-secreting cells (ASCs) triggered by immunization are globally recognized as CD19loCD38hiCD27hi. Yet, different vaccines give rise to antibody responses of different longevity, suggesting ASC populations are heterogeneous. We define circulating-ASC heterogeneity in vaccine responses using multicolor flow cytometry, morphology, VH repertoire, and RNA transcriptome analysis. We also tested differential survival using a human cell-free system that mimics the bone marrow (BM) microniche. In peripheral blood, we identified 3 CD19+ and 2 CD19- ASC subsets. All subsets contributed to the vaccine-specific responses and were characterized by in vivo proliferation and activation. The VH repertoire demonstrated strong oligoclonality with extensive interconnectivity among the 5 subsets and switched memory B cells. Transcriptome analysis showed separation of CD19+ and CD19- subsets that included pathways such as cell cycle, hypoxia, TNF-α, and unfolded protein response. They also demonstrated similar long-term in vitro survival after 48 days. In summary, vaccine-induced ASCs with different surface markers (CD19 and CD138) are derived from shared proliferative precursors yet express distinctive transcriptomes. Equal survival indicates that all ASC compartments are endowed with long-lived potential. Accordingly, in vivo survival of peripheral long-lived plasma cells may be determined in part by their homing and residence in the BM microniche.
Keywords: Adaptive immunity; Immunoglobulins; Immunology.
Conflict of interest statement
Figures





References
-
- Qian Y, et al. Elucidation of seventeen human peripheral blood B-cell subsets and quantification of the tetanus response using a density-based method for the automated identification of cell populations in multidimensional flow cytometry data. Cytometry B Clin Cytom. 2010;78 Suppl 1:S69–S82. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 TR001801/TR/NCATS NIH HHS/United States
- U19 AI109962/AI/NIAID NIH HHS/United States
- R01 AI121252/AI/NIAID NIH HHS/United States
- P01 AI125180/AI/NIAID NIH HHS/United States
- U19 AI056390/AI/NIAID NIH HHS/United States
- N01 AI050029/AI/NIAID NIH HHS/United States
- P01 AI078907/AI/NIAID NIH HHS/United States
- K23 AI067501/AI/NIAID NIH HHS/United States
- HHSN266200500030C/AI/NIAID NIH HHS/United States
- U01 AI141993/AI/NIAID NIH HHS/United States
- R37 AI049660/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U01 AI045969/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

