The HeartMate 6
- PMID: 31045916
- PMCID: PMC6925356
- DOI: 10.1097/MAT.0000000000001011
The HeartMate 6
Abstract
The need for biventricular support poses significant challenges for patients who require a mechanical bridge to transplantation. Recent improvements in ventricular assist device (VAD) technology has made possible the use of two centrifugal flow VADs as a total artificial heart (TAH) replacement. The HeartMate 3 (HM3; [Full MagLev, Abbott Laboratories, Chicago, Illinois]) was recently approved as a destination therapy; this VAD has a number of unique advantages that allow for its off-label use for biventricular support. Here, we describe the use of two HM3s as a TAH in a patient as a bridge to transplant.
Figures


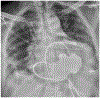
References
-
- Mozzafarian D et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 133, e38–e360 (2016). - PubMed
-
- Slaughter MS et al. HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009. 361 (23), 2241–51 (2009). - PubMed
-
- Fang JC Rise of the Machines — Left Ventricular Assist Devices as Permanent Therapy for Advanced Heart Failure N Engl J Med 361, 2282–85 (2009). - PubMed
-
- Kirklin JK et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 34 (12), 1495–504 (2015). - PubMed
-
- Leidenfrost J et al. Right ventricular assist device with membrane oxygenator support for right ventricular failure following implantable left ventricular assist device placement. Eur J Cardiothorac Surg. 49 (1), 73–7 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

