Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes
- PMID: 31046132
- PMCID: PMC6497129
- DOI: 10.1002/14651858.CD012611.pub3
Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes
Abstract
Background: One nutritional intervention advocated to prevent malnutrition among children is lipid-based nutrient supplements (LNS). LNS provide a range of vitamins and minerals, but unlike most other micronutrient supplements, LNS also provide energy, protein and essential fatty acids. Alternative recipes and formulations to LNS include fortified blended foods (FBF), which are foods fortified with vitamins and minerals, and micronutrient powders (MNP), which are a combination of vitamins and minerals, OBJECTIVES: To assess the effects and safety of preventive LNS given with complementary foods on health, nutrition and developmental outcomes of non-hospitalised infants and children six to 23 months of age, and whether or not they are more effective than other foods (including FBF or MNP).This review did not assess the effects of LNS as supplementary foods or therapeutic foods in the management of moderate and severe acute malnutrition.
Search methods: In October 2018, we searched CENTRAL, MEDLINE, Embase, 21 other databases and two trials registers for relevant studies. We also checked the reference lists of included studies and relevant reviews and contacted the authors of studies and other experts in the area for any ongoing and unpublished studies.
Selection criteria: Randomised controlled trials (RCTs) and quasi-RCTs that evaluated the impact of LNS plus complementary foods given at point-of-use (for any dose, frequency, duration) to non-hospitalised infants and young children aged six to 23 months in stable or emergency settings and compared to no intervention, other supplementary foods (i.e. FBF), nutrition counselling or multiple micronutrient supplements or powders for point-of-use fortification of complementary foods.
Data collection and analysis: Two review authors independently screened studies for relevance and, for those studies included in the review, extracted data, assessed risk of bias and rated the quality of the evidence using the GRADE approach. We carried out statistical analysis using Review Manager software. We used a random-effects meta-analysis for combining data as the interventions differed significantly. We set out the main findings of the review in 'Summary of findings' tables,.
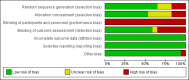
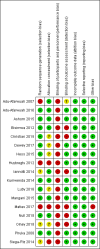
Main results: Our search identified a total of 8124 records, from which we included 17 studies (54 papers) with 23,200 children in the review. The included studies reported on one or more of the pre-specified primary outcomes, and five studies included multiple comparison groups.Overall, the majority of trials were at low risk of bias for random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias, but at high risk of bias for blinding of participants and personnel due to the nature of the intervention. Using the GRADE approach, we judged the quality of the evidence for most outcomes as low or moderate.LNS+complementary feeding compared with no intervention Thirteen studies compared LNS plus complementary feeding with no intervention. LNS plus complementary feeding reduced the prevalence of moderate stunting by 7% (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.88 to 0.98; nine studies, 13,372 participants; moderate-quality evidence), severe stunting by 15% (RR 0.85, 95% CI 0.74 to 0.98; five studies, 6151 participants; moderate-quality evidence), moderate wasting by 18% (RR 0.82, 95% CI 0.74 to 0.91; eight studies; 13,172 participants; moderate-quality evidence), moderate underweight by 15% (RR 0.85, 95% CI 0.80 to 0.91; eight studies, 13,073 participants; moderate-quality evidence), and anaemia by 21% (RR 0.79, 95% CI 0.69 to 0.90; five studies, 2332 participants; low-quality evidence). There was no impact of LNS plus complementary feeding on severe wasting (RR 1.27, 95% CI 0.66 to 2.46; three studies, 2329 participants) and severe underweight (RR 0.78, 95%CI 0.54 to 1.13; two studies, 1729 participants). Adverse effects did not differ between the groups (RR 0.86, 95% CI 0.74 to 1.01; three studies, 3382 participants).LNS+complementary feeding compared with FBF Five studies compared LNS plus complementary feeding with other FBF, including corn soy blend and UNIMIX. We pooled four of the five studies in meta-analyses and found that, when compared to other FBF, LNS plus complementary feeding significantly reduced the prevalence of moderate stunting (RR 0.89, 95% CI 0.82 to 0.97; three studies, 2828 participants; moderate-quality evidence), moderate wasting (RR 0.79, 95% CI 0.65 to 0.97; two studies, 2290 participants; moderate-quality evidence), and moderate underweight (RR 0.81, 95% CI 0.73 to 0.91; two studies, 2280 participants; moderate-quality evidence). We found no difference between LNS plus complementary feeding and FBF for severe stunting (RR 0.41, 95% CI 0.12 to 1.42; two studies, 729 participants; low-quality evidence), severe wasting (RR 0.64, 95% CI 0.19 to 2.81; two studies, 735 participants; moderate-quality evidence), and severe underweight (RR 1.23, 95% CI 0.67 to 2.25; one study, 173 participants; low-quality evidence).LNS+complementary feeding compared with MNP Four studies compared LNS plus complementary feeding with MNP. We pooled data from three of the four studies in meta-analyses and found that compared to MNP, LNS plus complementary feeding significantly reduced the prevalence of moderate underweight (RR 0.88, 95% CI 0.78 to 0.99; two studies, 2004 participants; moderate-quality evidence) and anaemia (RR 0.38, 95% CI 0.21 to 0.68; two studies, 557 participants; low-quality evidence). There was no difference between LNS plus complementary feeding and MNP for moderate stunting (RR 0.92, 95% CI 0.82 to 1.02; three studies, 2365 participants) and moderate wasting (RR 0.97, 95% CI 0.77 to 1.23; two studies, 2004 participants).
Authors' conclusions: The findings of this review suggest that LNS plus complementary feeding compared to no intervention is effective at improving growth outcomes and anaemia without adverse effects among children aged six to 23 months in low- and middle-income countries (LMIC) in Asia and Africa, and more effective if provided over a longer duration of time (over 12 months). Limited evidence also suggests that LNS plus complementary feeding is more effective than FBF and MNP at improving growth outcomes.
Conflict of interest statement
We certify that we have no affiliations with, or involvement in, any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
Jai K Das ‐ none known. Rehana A Salam ‐ none known. Yousaf Bashir Hadi ‐ none known. Sana Sadiq Sheikh ‐ none known. Afsah Zulfiqar Bhutta ‐ none known. Zita Weise Prinzo is a full‐time member of staff of the WHO. Zulfiqar A Bhutta's institution was awarded a grant from the WHO to undertake this review.
The review authors alone are responsible for the views expressed in this publication; the views do not necessarily represent the official position, decisions, policy or views of the WHO.
Figures



Update of
References
References to studies included in this review
Adu‐Afarwuah 2007 {published data only}
-
- Adu‐Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. American Journal of Clinical Nutrition 2008;87(4):929‐38. [DOI: 10.1093/ajcn/87.4.929; PUBMED: 18400716] - DOI - PubMed
-
- Adu‐Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. American Journal of Clinical Nutrition 2007;86(2):412‐20. [DOI: 10.1093/ajcn/86.2.412; PUBMED: 17684213] - DOI - PubMed
Adu‐Afarwuah 2016 {published data only}
-
- Adams KP, Lybbert TJ, Vosti SA, Ayifah E, Arimond M, Adu‐Afarwuah S, et al. Unintended effects of a targeted maternal and child nutrition intervention on household expenditures, labor income, and the nutritional status of non‐targeted siblings in Ghana. World Development 2018;107:138‐50. [DOI: 10.1016/j.worlddev.2018.02.025; PMC5917415; PUBMED: 29970953] - DOI - PMC - PubMed
-
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Ashorn U, Zeilani M, et al. Maternal supplementation with small‐quantity lipid‐based nutrient supplements compared with multiple micronutrients, but not with iron and folic acid, reduces the prevalence of low gestational weight gain in semi‐urban Ghana: a randomized controlled trial. Journal of Nutrition 2017;147(4):697‐705. [DOI: 10.3945/jn.116.242909; PMC5368579; PUBMED: 28275100] - DOI - PMC - PubMed
-
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Peerson JM, Arimond M, et al. Small‐quantity, lipid‐based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18‐mo‐old children in semi‐urban Ghana: a randomized controlled trial. American Journal of Clinical Nutrition 2016;104(3):797‐808. [DOI: 10.3945/ajcn.116.134692; PMC4997301; PUBMED: 27534634] - DOI - PMC - PubMed
-
- Adu‐Afarwuah S, Young RT, Lartey A, Okronipa H, Ashorn P, Ashorn U, et al. Supplementation during pregnancy with small‐quantity lipid‐based nutrient supplements or multiple micronutrients, compared with iron and folic acid, increases women's urinary iodine concentration in semiurban Ghana: a randomized controlled trial. Maternal & Child Nutrition 2018;14(2):e12570. [DOI: 10.1111/mcn.12570; PMC5900724; PUBMED: 29210520] - DOI - PMC - PubMed
Ashorn 2015 {published data only}
-
- Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Gondwe A, et al. Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small‐quantity lipid‐based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: a randomized controlled trial. Journal of Nutrition 2015;145(6):1345‐53. [DOI: 10.3945/jn.114.207225; PUBMED: 25926413] - DOI - PubMed
-
- Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Harjunmaa U, et al. The impact of lipid‐based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. American Journal of Clinical Nutrition 2015;101(2):387‐97. [DOI: 10.3945/ajcn.114.088617; PUBMED: 25646337] - DOI - PubMed
-
- Barua P, Chandrasiri UP, Beeson JG, Dewey KG, Maleta K, Ashorn P, et al. Effect of nutrient supplementation on the acquisition of humoral immunity to Plasmodium falciparum in young Malawian children. Malaria Journal 2018;17(1):74. [DOI: 10.1186/s12936-018-2224-6; PMC5804088; PUBMED: 29415730] - DOI - PMC - PubMed
-
- Jorgensen JM, Arnold C, Ashorn P, Ashorn U, Chaima D, Cheung YB, et al. Lipid‐based nutrient supplements during pregnancy and lactation did not affect human milk oligosaccharides and bioactive proteins in a randomized trial. Journal of Nutrition 2017;147(10):1867‐74. [DOI: 10.3945/jn.117.252981; PMC5610548; PUBMED: 28794206] - DOI - PMC - PubMed
Bisimwa 2012 {published data only}
-
- Bisimwa G, Owino VO, Bahwere P, Dramaix M, Donnen P, Dibari F, et al. Randomized controlled trial of the effectiveness of a soybean‐maize‐sorghum–based ready‐to‐use complementary food paste on infant growth in South Kivu, Democratic Republic of Congo. American Journal of Clinical Nutrition 2012;95(5):1157‐64. [DOI: 10.3945/ajcn.111.028704; PUBMED: 22492382] - DOI - PubMed
Christian 2015 {published data only}
-
- Christian P, Shaikh S, Shamim AA, Mehra S, Wu L, Mitra M, et al. Effect of fortified complementary food supplementation on child growth in rural Bangladesh: a cluster‐randomized trial. International Journal of Epidemiology 2015;44(6):1862‐76. [DOI: 10.1093/ije/dyv155; PMC4689999; PUBMED: 26275453] - DOI - PMC - PubMed
Dewey 2017 {published data only}
-
- Dewey KG, Mridha MK, Matias SL, Arnold CD, Cummins JR, Khan MS, et al. Lipid‐based nutrient supplementation in the first 1000 d improves child growth in Bangladesh: a cluster‐randomized effectiveness trial. American Journal of Clinical Nutrition 2017;105(4):944‐57. [DOI: 10.3945/ajcn.116.147942; PUBMED: 28275125] - DOI - PubMed
-
- Matias SL, Mridha MK, Tofail F, Arnold CD, Khan MS, Siddiqui Z, et al. Home fortification during the first 1000 d improves child development in Bangladesh: a cluster‐randomized effectiveness trial. American Journal of Clinical Nutrition 2017;105(4):958‐69. [DOI: 10.3945/ajcn.116.150318; PUBMED: 28275128] - DOI - PubMed
-
- Matias SL, Mridha MK, Young RT, Khan MS, Siddiqui Z, Ullah MB, et al. Prenatal and postnatal supplementation with lipid‐based nutrient supplements reduces anemia and iron deficiency in 18‐month‐old Bangladeshi children: a cluster‐randomized effectiveness trial. Journal of Nutrition 2018;148(7):1167‐76. [DOI: 10.1093/jn/nxy078; PUBMED: 29901736] - DOI - PubMed
Hess 2015 {published data only}
-
- Abbeddou S, Jimenez EY, Somé JW, Ouédraogo JB, Brown KH, Hess SY. Small‐quantity lipid‐based nutrient supplements containing different amounts of zinc along with diarrhea and malaria treatment increase iron and vitamin A status and reduce anemia prevalence, but do not affect zinc status in young Burkinabe children: a cluster‐randomized trial. BMC pediatrics 2017;17(1):46. [DOI: 10.1186/s12887-016-0765-9; PMC5288861; PUBMED: 28152989] - DOI - PMC - PubMed
-
- Hess SY, Abbeddou S, Jimenez EY, Ouédraogo JB, Brown KH. Iodine status of young Burkinabe children receiving small‐quantity lipid‐based nutrient supplements and iodised salt: a cluster‐randomised trial. British Journal of Nutrition 2015;114(11):1829‐37. [DOI: 10.1017/S0007114515003554; PUBMED: 26411504] - DOI - PubMed
-
- Hess SY, Abbeddou S, Jimenez EY, Somé JW, Vosti SA, Ouédraogo ZP, et al. Small‐quantity lipid‐based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young Burkinabe children: a cluster‐randomized trial. PLOS One 2015;10(3):e0122242. [DOI: 10.1371/journal.pone.0122242; PMC4376671; PUBMED: 25816354] - DOI - PMC - PubMed
-
- Prado EL, Abbeddou S, Jimenez EY, Somé JW, Ouédraogo ZP, Vosti SA, et al. Lipid‐based nutrient supplements plus malaria and diarrhea treatment increase infant development scores in a cluster‐randomized trial in Burkina Faso. Journal of Nutrition 2016;146(4):814‐22. [DOI: 10.3945/jn.115.225524; PUBMED: 26962193] - DOI - PubMed
-
- Somé JW, Abbeddou S, Jimenez EY, Hess SY, Ouédraogo ZP, Guissou RM, et al. Effect of zinc added to a daily small‐quantity lipid‐based nutrient supplement on diarrhoea, malaria, fever and respiratory infections in young children in rural Burkina Faso: a cluster‐randomised trial. BMJ Open 2015;5(9):e007828. [DOI: 10.1136/bmjopen-2015-007828; PMC4567679; PUBMED: 26362661] - DOI - PMC - PubMed
Huybregts 2012 {published data only}
-
- Huybregts L, Houngbé F, Salpéteur C, Brown R, Roberfroid D, Ait‐Aissa M, et al. The effect of adding ready‐to‐use supplementary food to a general food distribution on child nutritional status and morbidity: a cluster‐randomized controlled trial. PLOS Medicine 2012;9(9):e1001313. [DOI: 10.1371/journal.pmed.1001313; PMC3445445; PUBMED: 23028263] - DOI - PMC - PubMed
Iannotti 2014 {published data only}
-
- Iannotti LL, Dulience SJ, Green J, Joseph S, François J, Anténor ML, et al. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of a lipid‐based nutrient supplement. American Journal of Clinical Nutrition 2014;99(1):198‐208. [DOI: 10.3945/ajcn.113.063883; PMC3862455; PUBMED: 24225356] - DOI - PMC - PubMed
Kumwenda 2014 {published data only}
-
- Bendabenda J, Alho L, Ashorn U, Cheung YB, Dewey KG, Vosti SA, et al. The effect of providing lipid‐based nutrient supplements on morbidity in rural Malawian infants and young children: a randomized controlled trial. Public Health Nutrition 2016;19(10):1893‐903. [DOI: 10.1017/S1368980016000331; PUBMED: 26956611] - DOI - PMC - PubMed
-
- Maleta KM, Phuka J, Alho L, Cheung YB, Dewey KG, Ashorn U, et al. Provision of 10–40 g/d lipid‐based nutrient supplements from 6 to 18 months of age does not prevent linear growth faltering in Malawi. Journal of Nutrition 2015;145(8):1909‐15. [DOI: 10.3945/jn.114.208181; PUBMED: 26063066] - DOI - PubMed
-
- Prado EL, Phuka J, Maleta K, Ashorn P, Ashorn U, Vosti SA, et al. Provision of lipid‐based nutrient supplements from age 6 to 18 months does not affect infant development scores in a randomized trial in Malawi. Maternal and Child Health Journal 2016;20(10):2199‐208. [DOI: 10.1007/s10995-016-2061-6; PUBMED: 27395385] - DOI - PubMed
Luby 2018 {published data only}
-
- Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Global Health 2018;6(3):e302‐15. [DOI: 10.1016/S2214-109X(17)30490-4] - DOI - PMC - PubMed
-
- Parvez SM, Azad R, Rahman M, Unicomb L, Ram PK, Naser AM, et al. Achieving optimal technology and behavioral uptake of single and combined interventions of water, sanitation hygiene and nutrition, in an efficacy trial (WASH benefits) in rural Bangladesh. Trials 2018;19(1):358. [DOI: 10.1186/s13063-018-2710-8; PMC6034207; PUBMED: 29976251] - DOI - PMC - PubMed
-
- Tofail F, Fernald LC, Das KK, Rahman M, Ahmed T, Jannat KK, et al. Effect of water quality, sanitation, hand washing, and nutritional interventions on child development in rural Bangladesh (WASH Benefits Bangladesh): a cluster‐randomised controlled trial. Lancet Child & Adolescent Health 2018;2(4):255‐68. [DOI: 10.1016/S2352-4642(18)30031-2; PMC5859216; PUBMED: 29616235] - DOI - PMC - PubMed
Mangani 2015 {published data only}
-
- Aakko J, Grześkowiak Ł, Asukas T, Päivänsäde E, Lehto KM, Fan YM, et al. Lipid‐based nutrient supplements do not affect gut bifidobacterium microbiota in Malawian infants: a randomized trial. Journal of Pediatric Gastroenterology & Nutrition 2017;64(4):610‐5. [DOI: 10.1097/MPG.0000000000001333; PUBMED: 27403608] - DOI - PubMed
-
- Mangani C, Ashorn P, Maleta K, Phuka J, Thakwalakwa C, Dewey K, et al. Lipid‐based nutrient supplements do not affect the risk of malaria or respiratory morbidity in 6‐to 18‐month‐old Malawian children in a randomized controlled trial. Journal of Nutrition 2014;144(11):1835‐42. [DOI: 10.3945/jn.114.196139; PUBMED: 25332483] - DOI - PubMed
-
- Mangani C, Maleta K, Phuka J, Cheung YB, Thakwalakwa C, Dewey K, et al. Effect of complementary feeding with lipid‐based nutrient supplements and corn–soy blend on the incidence of stunting and linear growth among 6‐to 18‐month‐old infants and children in rural Malawi. Maternal & Child Nutrition 2015;11(Suppl 4):132‐43. [DOI: 10.1111/mcn.12068; PUBMED: 23795976] - DOI - PMC - PubMed
Matias 2017 {published data only}
-
- Matias SL, Vargas‐Vásquez A, Pérez RB, Valdivia LA, Vivanco OA, Martín AR, et al. Effects of lipid‐based nutrient supplements v micronutrient powders on nutritional and developmental outcomes among Peruvian infants. Public Health Nutrition 2017;20(16):2998‐3007. [DOI: 10.1017/S1368980017001811; PUBMED: 28789712] - DOI - PMC - PubMed
Null 2018 {published data only}
-
- Byrd K, Dentz HN, Williams A, Kiprotich M, Pickering AJ, Omondi R, et al. A behaviour change intervention with lipid‐based nutrient supplements had little impact on young child feeding indicators in rural Kenya. Maternal & Child Nutrition 2019;15(1):e12660. [DOI: 10.1111/mcn.12660; PUBMED: 30207423] - DOI - PMC - PubMed
-
- Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster‐randomised controlled trial. Lancet Global Health 2018;6(3):e316‐29. [DOI: 10.1016/S2214-109X(18)30005-6; PMC5809717; PUBMED: 29396219] - DOI - PMC - PubMed
-
- Stewart CP, Kariger P, Fernald L, Pickering AJ, Arnold CD, Arnold BF, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on child development in rural Kenya (WASH Benefits Kenya): a cluster‐randomised controlled trial. Lancet Child & Adolescent Health 2018;2(4):269‐80. - PMC - PubMed
Olney 2018 {published data only}
-
- Olney DK, Leroy J, Bliznashka L, Ruel MT. PROCOMIDA, a food‐assisted maternal and child health and nutrition program, reduces child stunting in Guatemala: a cluster‐randomized controlled intervention trial. Journal of Nutrition 2018;148(9):1493‐505. [DOI: 10.1093/jn/nxy138; PMC6118165; PUBMED: 30184223] - DOI - PMC - PubMed
Phuka 2008 {published data only}
-
- Phuka JC, Gladstone M, Maleta K, Thakwalakwa C, Cheung YB, Briend A, et al. Developmental outcomes among 18‐month‐old Malawians after a year of complementary feeding with lipid‐based nutrient supplements or corn‐soy flour. Maternal & Child Nutrition 2012;8(2):239‐48. [DOI: 10.1111/j.1740-8709.2011.00294.x; PUBMED: 21342456] - DOI - PMC - PubMed
-
- Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, et al. Complementary feeding with fortified spread and incidence of severe stunting in 6‐to 18‐month‐old rural Malawians. Archives of Pediatrics & Adolescent Medicine 2008;162(7):619‐26. [DOI: 10.1001/archpedi.162.7.619; PMC3721756; PUBMED: 18606932] - DOI - PMC - PubMed
-
- Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, et al. Postintervention growth of Malawian children who received 12‐mo dietary complementation with a lipid‐based nutrient supplement or maize‐soy flour. American Journal of Clinical Nutrition 2009;89(1):382‐90. [DOI: 10.3945/ajcn.2008.26483; PUBMED: 19056572] - DOI - PubMed
Siega‐Riz 2014 {published data only}
-
- Flax VL, Siega‐Riz AM, Reinhart GA, Bentley ME. Provision of lipid‐based nutrient supplements to Honduran children increases their dietary macro‐ and micronutrient intake without displacing other foods. Maternal & Child Nutrition 2015;11(Suppl 4):203‐13. [DOI: 10.1111/mcn.12182; PUBMED: 25819697] - DOI - PMC - PubMed
-
- Siega‐Riz AM, Estrada Del Campo Y, Kinlaw A, Reinhart GA, Allen LH, Shahab‐Ferdows S, et al. Effect of supplementation with a lipid‐based nutrient supplement on the micronutrient status of children aged 6–18 months living in the rural region of Intibucá, Honduras. Paediatric and Perinatal Epidemiology 2014;28(3):245‐54. [DOI: 10.1111/ppe.12117; PUBMED: 24628577] - DOI - PMC - PubMed
References to studies excluded from this review
Ackatia‐Armah 2015 {published data only}
-
- Ackatia‐Armah RS, McDonald CM, Doumbia S, Erhardt JG, Hamer DH, Brown KH. Malian children with moderate acute malnutrition who are treated with lipid‐based dietary supplements have greater weight gains and recovery rates than those treated with locally produced cereal‐legume products: a community‐based, cluster‐randomized trial. American Journal of Clinical Nutrition 2015;101(3):632‐45. [DOI: 10.3945/ajcn.113.069807; PUBMED: 25733649] - DOI - PubMed
Adams 2017 {published data only}
-
- Adams KP, Ayifah E, Phiri TE, Mridha MK, Adu‐Afarwuah S, Arimond M, et al. Maternal and child supplementation with lipid‐based nutrient supplements, but not child supplementation alone, decreases self‐reported household food insecurity in some settings. Journal of Nutrition 2017;147(12):2309‐18. [DOI: 10.3945/jn.117.257386; PMC5697970; PUBMED: 28978680] - DOI - PMC - PubMed
Adams 2018 {published data only}
-
- Adams KP, Vosti SA, Ayifah E, Phiri TE, Adu‐Afarwuah S, Maleta K, et al. Willingness to pay for small‐quantity lipid‐based nutrient supplements for women and children: evidence from Ghana and Malawi. Maternal & Child Nutrition 2018;14(2):e12518. [DOI: 10.1111/mcn.12518; PMC6088232; PUBMED: 28960913] - DOI - PMC - PubMed
Ahmed 2014 {published data only}
-
- Ahmed T, Choudhury N, Hossain MI, Tangsuphoom N, Islam MM, Pee S, et al. Development and acceptability testing of ready‐to‐use supplementary food made from locally available food ingredients in Bangladesh. BMC Pediatrics 2014;14:164. [DOI: 10.1186/1471-2431-14-164; PMC4098698; PUBMED: 24972632] - DOI - PMC - PubMed
Arimond 2017 {published data only}
-
- Arimond M, Abbeddou S, Kumwenda C, Okronipa H, Hemsworth J, Jimenez EY, et al. Impact of small quantity lipid‐based nutrient supplements on infant and young child feeding practices at 18 months of age: results from four randomized controlled trials in Africa. Maternal & Child Nutrition 2017;13(3):e12377. [DOI: 10.1111/mcn.12377; PMC5516197; PUBMED: 27910260] - DOI - PMC - PubMed
Cercamondi 2013 {published data only}
-
- Cercamondi CI, Egli IM, Mitchikpe E, Tossou F, Hessou J, Zeder C, et al. Iron bioavailability from a lipid‐based complementary food fortificant mixed with millet porridge can be optimized by adding phytase and ascorbic acid but not by using a mixture of ferrous sulfate and sodium iron EDTA. Journal of Nutrition 2013;143(8):1233‐9. [DOI: 10.3945/jn.113.175075; PUBMED: 23761652] - DOI - PubMed
Defourney 2009 {published data only}
-
- Defourny I, Minetti A, Harczi G, Doyon S, Shepherd S, Tectonidis M, et al. A large‐scale distribution of milk‐based fortified spreads: evidence for a new approach in regions with high burden of acute malnutrition. PLOS One 2009;4(5):e5455. [DOI: 10.1371/journal.pone.0005455; PMC2673585; PUBMED: 19421316] - DOI - PMC - PubMed
Flax 2010 {published data only}
-
- Flax VL, Phuka J, Cheung YB, Ashorn U, Maleta K, Ashorn P. Feeding patterns and behaviors during home supplementation of underweight Malawian children with lipid‐based nutrient supplements or corn‐soy blend. Appetite 2010;54(3):504‐11. [DOI: 10.1016/j.appet.2010.02.003; PUBMED: 20153389] - DOI - PubMed
Flax 2013 {published data only}
-
- Flax VL, Bentley ME, Chasela CS, Kayira D, Hudgens MG, Kacheche KZ, et al. Lipid‐based nutrient supplements are feasible as a breastmilk replacement for HIV‐exposed infants from 24 to 48 weeks of age. Journal of Nutrition 2013;143(5):701‐7. [DOI: 10.3945/jn.112.168245; PMC3738238; PUBMED: 23468553] - DOI - PMC - PubMed
Heidkamp 2012 {published data only}
-
- Heidkamp RA, Stoltzfus RJ, Fitzgerald DW, Pape JW. Growth in late infancy among HIV‐exposed children in urban Haiti is associated with participation in a clinic‐based infant feeding support intervention. Journal of Nutrition 2012;142(4):774‐80. [DOI: 10.3945/jn.111.155275; PMC3301993; PUBMED: 22378328] - DOI - PMC - PubMed
Isanaka 2009 {published data only}
-
- Isanaka S, Nombela N, Djibo A, Poupard M, Beckhoven D, Gaboulaud V, et al. Effect of preventive supplementation with ready‐to‐use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. JAMA 2009;301(3):277‐85. [DOI: 10.1001/jama.2008.1018; PMC3144630 ; PUBMED: 19155454] - DOI - PMC - PubMed
Iuel‐Brockdorf 2015 {published data only}
-
- Iuel‐Brockdorf AS, Dræbel TA, Fabiansen C, Cichon B, Christensen VB, Yameogo C, et al. Acceptability of new formulations of corn‐soy blends and lipid‐based nutrient supplements in Province du Passoré, Burkina Faso. Appetite 2015;91(1):278‐86. [DOI: 10.1016/j.appet.2015.04.058; PUBMED: 25913687] - DOI - PubMed
Kuusipalo 2006 {published data only}
-
- Kuusipalo H, Maleta K, Briend A, Manary M, Ashorn P. Growth and change in blood haemoglobin concentration among underweight Malawian infants receiving fortified spreads for 12 weeks: a preliminary trial. Journal of Pediatric Gastroenterology and Nutrition 2006;43(4):525‐32. [DOI: 10.1097/01.mpg.0000235981.26700.d3; PUBMED: 17033530] - DOI - PubMed
LaGrone 2012 {published data only}
-
- LaGrone LN, Trehan I, Meuli GJ, Wang RJ, Thakwalakwa C, Maleta K, et al. A novel fortified blended flour, corn‐soy blend “plus‐plus,” is not inferior to lipid‐based ready‐to‐use supplementary foods for the treatment of moderate acute malnutrition in Malawian children. American Journal of Clinical Nutrition 2012;95(1):212‐9. [DOI: 10.3945/ajcn.111.022525; PMC3238461; PUBMED: 22170366] - DOI - PMC - PubMed
Langendorf 2014 {published data only}
-
- Prudhon C, Langendorf C, Roederer T, Doyon S, Mamaty AA, Woi‐Messe L, et al. Effect of ready‐to‐use foods for preventing child undernutrition in Niger: analysis of a prospective intervention study over 15 months of follow‐up. Maternal & Child Nutrition 2017;13(1):e12236. [DOI: 10.1111/mcn.12236; PUBMED: 26775560] - DOI - PMC - PubMed
-
- Sayyad‐Neerkorn J, Langendorf C, Roederer T, Doyon S, Mamaty AA, Woi‐Messe L, et al. Preventive effects of long‐term supplementation with 2 nutritious food supplements in young children in Niger. Journal of Nutrition 2015;145(11):2596‐603. [DOI: 10.3945/jn.115.213157; PUBMED: 26423742] - DOI - PubMed
Maleta 2004 {published data only}
-
- Maleta K, Kuittinen J, Duggan MB, Briend A, Manary M, Wales J, et al. Supplementary feeding of underweight, stunted Malawian children with a ready‐to‐use food. Journal of Pediatric Gastroenterology and Nutrition 2004;38(2):152‐8. [PUBMED: 14734876] - PubMed
Maryam 2015 {published data only}
-
- Maryam A, Hadju V, As'ad S, Bahar B. Factors related to the status of vitamin A and zink baduta aged 6‐23 months in the district of East Central South, Province of Nusa Tenggara Timur. International Journal of Sciences: Basic and Applied Research 2015;23(2):156‐63. [gssrr.org/index.php?journal=JournalOfBasicAndApplied&page=article&am...
Muslihah 2016 {published data only}
-
- Muslihah N, Khomsan A, Briawan D, Riyadi H. Effect of the provision of small‐quantity lipid‐based nutrient supplements on gross motor developmental milestones in Indonesian infants. Pakistan Journal of Nutrition 2016;15(9):889‐96. [DOI: 10.3923/pjn.2016.889.896] - DOI
Rantesalu 2017 {published data only}
-
- Rantesalu M. Effect of a lipid‐based nutrient supplement on insulin‐like growth factor‐1 level (IGF‐1) in 6‐12 month‐old children in South Central Timor of the East Nusa Tenggara Province. Pakistan Journal of Nutrition 2017;16(6):417‐25. [DOI: 10.3923/pjn.2017.417.425] - DOI
Schlossman 2017 {published data only}
-
- Schlossman N, Brown C, Batra P, Sa AB, Balan I, Balan A, et al. A randomized controlled trial of two ready‐to‐use supplementary foods demonstrates benefit of the higher dairy supplement for reduced wasting in mothers, and differential impact in infants and children associated with maternal supplement response. Food and Nutrition Bulletin 2017;38(3):275‐90. [DOI: 10.1177/0379572117700754; PUBMED: 28374648] - DOI - PubMed
Style 2017 {published data only}
-
- Style S, Tondeur M, Grijalva‐Eternod C, Pringle J, Kassim I, Wilkinson C, et al. Assessment of the effectiveness of a small quantity lipid‐based nutrient supplement on reducing anaemia and stunting in refugee populations in the Horn of Africa: secondary data analysis. PLOS One 2017;12(6):e0177556. [DOI: 10.1371/journal.pone.0177556; PMC5462343; PUBMED: 28591166] - DOI - PMC - PubMed
Thakwalakwa 2010 {published data only}
-
- Thakwalakwa C, Ashorn P, Phuka J, Cheung YB, Briend A, Puumalainen T, et al. A lipid‐based nutrient supplement but not corn‐soy blend modestly increases weight gain among 6‐ to 18‐month‐old moderately underweight children in rural Malawi. Journal of Nutrition 2010;140(11):2008‐13. [DOI: 10.3945/jn.110.122499; PUBMED: 20861218] - DOI - PubMed
Thakwalakwa 2012 {published data only}
-
- Thakwalakwa CM, Ashorn P, Jawati M, Phuka JC, Cheung YB, Maleta KM. An effectiveness trial showed lipid‐based nutrient supplementation but not corn–soya blend offered a modest benefit in weight gain among 6‐to 18‐month‐old underweight children in rural Malawi. Public Health Nutrition 2012;15(9):1755‐62. [DOI: 10.1017/S1368980012003023; PUBMED: 22691922] - DOI - PubMed
Thakwalakwa 2015 {published data only}
-
- Thakwalakwa CM, Ashorn P, Phuka JC, Cheung YB, Briend A, Maleta KM. Impact of lipid‐based nutrient supplements and corn–soy blend on energy and nutrient intake among moderately underweight 8–18‐month‐old children participating in a clinical trial. Maternal & Child Nutrition 2015;11(Suppl 4):144‐50. [DOI: 10.1111/mcn.12105; PUBMED: 24528807] - DOI - PMC - PubMed
Unger 2017 {published data only}
-
- Unger SA, Drammeh S, Hasan J, Ceesay K, Sinjanka E, Beyai S, et al. Impact of fortified versus unfortified lipid‐based supplements on morbidity and nutritional status: a randomised double‐blind placebo‐controlled trial in ill Gambian children. PLOS Medicine 2017;14(8):e1002377. [DOI: 10.1371/journal.pmed.1002377; PMC5557358; PUBMED: 28809926] - DOI - PMC - PubMed
Vargas‐Vásquez 2015 {published data only}
-
- Vargas‐Vásquez A, Bado R, Alcázar L, Aquino O, Rodríguez A, Novalbos JP. Effects of a lipid‐based nutrient supplement on hemoglobin levels and anthropometric indicators in children from five districts in Huánuco Peru [Efecto de un suplemento nutricional a base de lípidos en los niveles de hemoglobina e indicadores antropométricos en niños de cinco distritos de Huánuco, Perú]. Revista Peruana de Medicina Experimental y Salud Pública 2015;32(2):237‐44. [PUBMED: 26338380] - PubMed
References to ongoing studies
Borg 2017 {published data only}
-
- Borg B, Mihrshahi S, Griffin M, Chamnan C, Laillou A, Wieringa FT. Crossover trial to test the acceptability of a locally produced lipid‐based nutrient supplement (LNS) for children under 2 years in Cambodia: a study protocol. BMJ Open 2017;7(9):e015958. [DOI: 10.1136/bmjopen-2017-015958] - DOI - PMC - PubMed
Fernald 2016 {published data only}
-
- Fernald LC, Galasso E, Qamruddin J, Ranaivoson C, Ratsifandrihamanana L, Stewart CP, et al. A cluster‐randomized, controlled trial of nutritional supplementation and promotion of responsive parenting in Madagascar: the MAHAY study design and rationale. BMC Public Health 2016;16:466. [10.1186/s12889‐016‐3097‐7] - PMC - PubMed
Huybregts 2017 {published data only}
-
- Huybregts L, Becquey E, Zongrone A, Port A, Khassanova R, Coulibaly L, et al. The impact of integrated prevention and treatment on child malnutrition and health: the PROMIS project, a randomized control trial in Burkina Faso and Mali. BMC Public Health 2017;17(1):237. [DOI: 10.1186/s12889-017-4146-6; PMC5343313; PUBMED: 28274214] - DOI - PMC - PubMed
ISRCTN94319790 {published data only}
-
- ISRCTN94319790. Ready to Use Supplementary Foods (RUSF) to prevent stunting among children under five years in Kurram Agency [A community‐based cluster controlled trial to evaluate the effectiveness of Ready to Use Supplementary Foods (RUSF) and proportional contribution of multi‐sectoral interventions in the prevention of stunting among children under five years in Kurram Agency, Pakistan]. apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN94319790 (first received 11 December 2017).
SHINE trial 2015 {published data only}
-
- Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial Team, Humphrey JH, Jones AD, Manges A, Mangwadu G, Maluccio JA, Mbuya MN, et al. The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial: rationale, design, and methods. Clinical Infectious Diseases 2015;61(Supp 7):S685‐702. [DOI: 10.1093/cid/civ844; PMC4657589; PUBMED: 26602296] - DOI - PMC - PubMed
Additional references
Adu‐Afarwuah 2011
Arimond 2015
-
- Arimond M, Zeilani M, Jungjohann S, Brown KH, Ashorn P, Allen LH, et al. Considerations in developing lipid‐based nutrient supplements for prevention of undernutrition: experience from the International Lipid‐Based Nutrient Supplements (iLiNS) Project. Maternal & Child Nutrition 2015;11 Suppl 4:31‐61. [DOI: 10.1111/mcn.12049; PUBMED: 23647784] - DOI - PMC - PubMed
Balshem 2010
Bendabenda 2016
-
- Bendabenda J, Alho L, Ashorn U, Cheung YB, Dewey KG, Vosti SA, et al. The effect of providing lipid‐based nutrient supplements on morbidity in rural Malawian infants and young children: a randomized controlled trial. Public Health Nutrition 2016;19(10):1893‐903. [DOI: 10.1017/S1368980016000331; PUBMED: 26956611] - DOI - PMC - PubMed
Bhutta 2013
Black 2013
Chaparro 2010
-
- Chaparro CM, Dewey KG. Use of lipid‐based nutrient supplements (LNS) to improve the nutrient adequacy of general food distribution rations for vulnerable sub‐groups in emergency settings. Maternal & Child Nutrition 2010;6(Suppl 1):1‐69. [DOI: 10.1111/j.1740-8709.2009.00224.x; PUBMED: 20055936] - DOI - PMC - PubMed
Das 2018
-
- Das JK, Hoodbhoy Z, Salam RA, Bhutta AZ, Valenzuela‐Rubio NG, Weise Prinzo Z, et al. Lipid‐based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD012610.pub2; PUBMED: 30168868] - DOI - PMC - PubMed
De‐Regil 2013
-
- De‐Regil LM, Suchdev PS, Vist GE, Walleser S, Peña‐Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review). Evidence‐Based Child Health: A Cochrane Review Journal 2013;8(1):112‐201. [DOI: 10.1002/ebch.1895] - DOI - PubMed
Deeks 2011
-
- Deeks J, Higgins JP, Altman D on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dewey 2012
Dewey 2016 [pers comm]
-
- Dewey KG. RE: Preliminary results of LNS reviews [personal communication]. Email to: ZA Bhutta 14 November 2016.
Dewey 2017 [pers comm]
-
- Dewey KG. Another iLiNS‐ZINC paper [personal communication]. Email to: ZA Bhutta 04 February 2017.
Egger 1997
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 29 April 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Harbord 2006
Hess 2011
-
- Hess SY, Bado L, Aaron GJ, Ouédraogo JB, Zeilani M, Brown KH. Acceptability of zinc‐fortified, lipid‐based nutrient supplements (LNS) prepared for young children in Burkina Faso. Maternal & Child Nutrition 2011;7(4):357‐67. [DOI: 10.1111/j.1740-8709.2010.00287.x; PUBMED: 21159124] - DOI - PMC - PubMed
Higgins 2011a
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Iannotti 2013
-
- Iannotti LL, Dulience SJ, Green J, Joseph S, François J, Anténor ML, et al. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of a lipid‐based nutrient supplement. Amercian Journal of Clinical Nutrition 2013;99(1):198‐208. [DOI: 10.3945/%E2%80%8Bajcn.113.063883] - DOI - PMC - PubMed
Ilins 2015
-
- iLiNS. iLiNS Project. ilins.org/resources (accessed 12 January 2016).
Kristjansson 2015
-
- Kristjansson E, Francis DK, Liberato S, Benkhalti Jandu M, Welch V, Batal M, et al. Food supplementation for improving the physical and psychosocial health of socio‐economically disadvantaged children aged three months to five years. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD009924.pub2; PUBMED: 25739460] - DOI - PMC - PubMed
Kristjansson 2016
-
- Kristjansson E, Francis D, Liberato S, Greenhalgh T, Welch V, Jandu MB, et al. Supplementary Feeding for Improving the Health of Disadvantaged Infants and Children: What Works and Why? Systematic Review Summary 5. London (UK): International Initiative for Impact Evaluation (3ie), 2016.
Larson 2017
Lassi 2013
-
- Lassi ZS, Das JK, Zahid G, Imdad A, Bhutta ZA. Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: a systematic review. BMC Public Health 2013;13(Suppl 3):S13. [DOI: 10.1186/1471-2458-13-S3-S13; PMC3847349; PUBMED: 24564534] - DOI - PMC - PubMed
Liu 2012
Maleta 2015
-
- Maleta KM, Phuka J, Alho L, Cheung YB, Dewey KG, Ashorn U, et al. Provision of 10–40 g/d lipid‐based nutrient supplements from 6 to 18 months of age does not prevent linear growth faltering in Malawi. Journal of Nutrition 2015;145(8):1909‐15. [DOI: 10.3945/jn.114.208181; PUBMED: 26063066] - DOI - PubMed
Mangani 2013
Moher 2009
Pee 2008
-
- Pee S, Bloem MW. Current and potential role of specially formulated foods and food supplements for preventing malnutrition among 6‐23 months old and treating moderate malnutrition among 6‐59 months old children. www.who.int/nutrition/publications/moderate_malnutrition/MM_Background_p... (accessed 14 December 2016). - PubMed
Prado 2016
-
- Prado EL, Maleta K, Ashorn P, Ashorn U, Vosti SA, Sadalaki J, et al. Effects of maternal and child lipid‐based nutrient supplements on infant development: a randomized trial in Malawi. American Journal of Clinical Nutrition 2016;103(3):784‐93. [DOI: 10.3945/ajcn.115.114579; PUBMED: 26843155] - DOI - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Segrè 2015
Sguassero 2012
-
- Sguassero Y, Onis M, Bonotti AM, Carroli G. Community‐based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD005039.pub3; PUBMED: 22696347] - DOI - PMC - PubMed
Stewart 2017 [pers comm]
-
- Stewart CP. RE: [personal communication]. Email to: ZA Bhutta 27 April 2017.
The World Bank 2006
-
- The World Bank. Repositioning Nutrition as Central to Development: A Strategy for Large Scale Action. Overview. Washington (DC): The World Bank, 2006. [siteresources.worldbank.org/NUTRITION/Resources/281846‐1131636806329/Nut...
UNICEF 2013
-
- UNICEF, CDC. Global assessment of home fortification interventions, 2011: final report May 2013. www.hftag.org/resource/global‐assessment‐of‐home‐fortification‐intervent... (accessed 21 November 2015).
UNICEF 2014
-
- UNICEF. Humanitarian action for children 2014: overview. www.unicef.org/gambia/Humanitarian_Action_for_Childen_2014_Overview.pdf (accessed 22 November 2015).
Victora 2008
WHO 2011
-
- World Health Organization. Guideline: use of multiple micronutrient powders for home fortification of foods consumed by infants and children 6–23 months of age. apps.who.int/iris/bitstream/10665/44651/1/9789241502047_eng.pdf (accessed 25 November 2015).
WHO 2012
-
- WHO. Technical Note: Supplementary Foods for the Management of Moderate Acute Malnutrition in Infants and Children 6‐59 Months of Age. Geneva (CH): World Health Organization, 2012. [apps.who.int/iris/bitstream/10665/75836/1/9789241504423_eng.pdf]
WHO 2013
-
- World Health Organization. Guideline: updates on the management of severe acute malnutrition in infants and children. apps.who.int/iris/bitstream/10665/95584/1/9789241506328_eng.pdf (accessed 4 December 2015). - PubMed
WHO 2014
-
- World Health Organization. WHA global nutrition targets 2025: stunting policy brief. www.who.int/nutrition/topics/globaltargets_stunting_policybrief.pdf (accessed 4 January 2016).
Wisner 2002
-
- Wisner B, Adams J, editor(s). Environmental Health in Emergencies and Disasters: A Practical Guide. Geneva (CH): World Health Organization, 2002. [apps.who.int/iris/bitstream/handle/10665/42561/9241545410_eng.pdf;jsessi...
References to other published versions of this review
Das 2017
-
- Das JK, Salam RA, Weise Prinzo Z, Sadiq Sheikh S, Bhutta ZA. Provision of preventive lipid‐based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD012611] - DOI - PMC - PubMed
Das 2019
-
- Das JK, Salam RA, Weise Prinzo Z, Sadiq Sheikh S, Bhutta ZA. Provision of preventive lipid‐based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database of Systematic Reviews 2019, Issue 3. [DOI: 10.1002/14651858.CD012611.pub2] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

