Cobalt-Catalyzed Intramolecular Silylperoxidation of Unsaturated Diisopropylsilyl Ethers
- PMID: 31046281
- PMCID: PMC7189782
- DOI: 10.1021/acs.joc.9b00642
Cobalt-Catalyzed Intramolecular Silylperoxidation of Unsaturated Diisopropylsilyl Ethers
Abstract
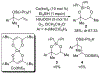
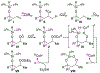
A cobalt-catalyzed intramolecular silylperoxidation reaction was developed that allows for the conversion of unsaturated diisopropylsilyl ethers to 3-sila-1,2,4-trioxepanes. Reduction of the peroxide unit of the 3-sila-1,2,4-trioxepane yields six-membered ring diisopropylsilylene acetals.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

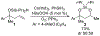
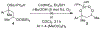

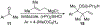

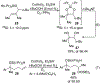
References
-
- Shigeru I; Teruaki M Novel Method for the Preparation of Triethylsilyl Peroxides from Olefins by the Reaction with Molecular Oxygen and Triethylsilane Catalyzed by Bis(1,3-diketonato)cobalt(II). Chem. Lett 1989, 18, 573–576.
-
- Tokuyasu T; Kunikawa S; Masuyama A; Nojima M Co(III)–Alkyl Complex- and Co(III)–Alkylperoxo Complex-Catalyzed Triethylsilylperoxidation of Alkenes with Molecular Oxygen and Triethylsilane. Org. Lett 2002, 4, 3595–3598. - PubMed
-
- Tokuyasu T; Kunikawa S; McCullough KJ; Masuyama A; Nojima M Synthesis of Cyclic Peroxides by Chemo- and Regioselective Peroxidation of Dienes with Co(II)/O2/Et3SiH. J. Org. Chem 2005, 70, 251–260. - PubMed
-
- Hurlocker B; Miner MR; Woerpel KA Synthesis of Silyl Monoperoxyketals by Regioselective Cobalt-Catalyzed Peroxidation of Silyl Enol Ethers: Application to the Synthesis of 1,2-Dioxolanes. Org. Lett 2014, 16, 4280–4283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

