Influenza epidemiology and influenza vaccine effectiveness during the 2016-2017 season in the Global Influenza Hospital Surveillance Network (GIHSN)
- PMID: 31046725
- PMCID: PMC6498567
- DOI: 10.1186/s12889-019-6713-5
Influenza epidemiology and influenza vaccine effectiveness during the 2016-2017 season in the Global Influenza Hospital Surveillance Network (GIHSN)
Abstract
Background: The Global Influenza Hospital Surveillance Network (GIHSN) aims to determine the burden of severe influenza disease and Influenza Vaccine Effectiveness (IVE). This is a prospective, active surveillance and hospital-based epidemiological study to collect epidemiological data in the GIHSN. In the 2016-2017 influenza season, 15 sites in 14 countries participated in the GIHSN, although the analyses could not be performed in 2 sites. A common core protocol was used in order to make results comparable. Here we present the results of the GIHSN 2016-2017 influenza season.
Methods: A RT-PCR test was performed to all patients that accomplished the requirements detailed on a common core protocol. Patients admitted were included in the study after signing the informed consent, if they were residents, not institutionalised, not discharged in the previous 30 days from other hospitalisation with symptoms onset within the 7 days prior to admission. Patients 5 years old or more must also complied the Influenza-Like Illness definition. A test negative-design was implemented to perform IVE analysis. IVE was estimated using a logistic regression model, with the formula IVE = (1-aOR) × 100, where aOR is the adjusted Odds Ratio comparing cases and controls.
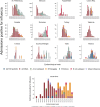
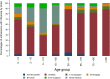
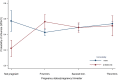
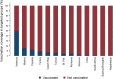
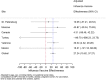
Results: Among 21,967 screened patients, 10,140 (46.16%) were included, as they accomplished the inclusion criteria, and tested, and therefore 11,827 (53.84%) patients were excluded. Around 60% of all patients included with laboratory results were recruited at 3 sites. The predominant strain was A(H3N2), detected in 63.6% of the cases (1840 patients), followed by B/Victoria, in 21.3% of the cases (618 patients). There were 2895 influenza positive patients (28.6% of the included patients). A(H1N1)pdm09 strain was mainly found in Mexico. IVE could only be performed in 6 sites separately. Overall IVE was 27.24 (95% CI 15.62-37.27. Vaccination seemed to confer better protection against influenza B and in people 2-4 years, or 85 years old or older. The aOR for hospitalized and testing positive for influenza was 3.02 (95% CI 1.59-5.76) comparing pregnant with non-pregnant women.
Conclusions: Vaccination prevented around 1 in 4 hospitalisations with influenza. Sparse numbers didn't allow estimating IVE in all sites separately. Pregnancy was found a risk factor for influenza, having 3 times more risk of being admitted with influenza for pregnant women.
Keywords: Epidemiology; Influenza virus; Surveillance; Vaccine effectiveness.
Conflict of interest statement
Ethics approval and consent to participate
This study has been approved by the Ethics Committees of the participating sites, who have approved their participation in the GIHSN network. Each adult patient tested for influenza had signed an informed consent in order to be included in the study. In case the patient did not reach the legal age or is impaired, parents or legal guardians signed the informed consent. The Ethics Committees of the participating sites are listed below:
St. Petersburg: Local Ethical Committee under the FGBU “Research Institute of Influenza” of the Ministry of Health of the Russian Federation
Moscow: The local Ethic Committee of Hospital #1 for Infectious Diseases of Moscow Health Department
Kazakhstan: The study was carried in Almaty, Kazakhstan as part of the implementation of the national Severe Acute Respiratory Infections (SARI) surveillance program in Kazakhstan for purposes of communicable disease control. Ethical approval was not required but informed consent was obtained before inclusion. Informed consent provided in accordance with the Constitution of the Republic of Kazakhstan (section II article 29)
Czech Republic: Ethics Committee of the Hospital Na Bulovce
Canada: The Nova Scotia Health Authority Research Ethics Board and the IWK Research Ethics Board
(IWK: Isaak Walton Killam) Romania: Bioethics Committee of the National Institute for Infectious Diseases “Prof. Dr. Matei Bals” Bucharest, Romania
Turkey: Hacettepe University Non-interventional Clinical Research Ethics Board
Valencia: Comité Ético de Investigación Clínica Dirección General de Salud Pública-Centro Superior de Investigación en Salud Pública (CEIC-DGSP-CSISP)
Tunisia: The ethics committee of Abderrahmane Mami hospital, Ariana, Tunisia
Suzhou/Shanghai: Fudan University School of Public Health Institutional Review Board
India: Institutional Ethics Committee of the Sher-i-Kashmir Institute of Medical Sciences, Srinagar
Mexico: Research Ethics Committee of the National Institute of Medical Science and Nutrition Salvador Zubiran & Research Committee of the National Institute of Medical Science and Nutrition Salvador Zubiran
South Africa: The Human Research Ethics Committee of the University of the Witwatersrand
All of these Ethics Committees approved the participation of the site in the study and the data transfer to FISABIO, who led the implementation and data collection in the 2016–2017 season.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures







References
-
- Puig-Barbera J, Burtseva E, Yu H, Cowling BJ, Badur S, Kyncl J, et al. Influenza epidemiology and influenza vaccine effectiveness during the 2014-2015 season: annual report from the global influenza hospital surveillance network. BMC Public Health. 2016;16(Suppl 1):757. doi: 10.1186/s12889-016-3378-1. - DOI - PMC - PubMed
-
- Puig-Barberà J, Natividad-Sancho A, Trushakova S, Sominina A, Pisareva M, Ciblak MA, et al. Epidemiology of hospital admissions with influenza during the 2013/2014 northern hemisphere influenza season: results from the global influenza hospital surveillance network. PLoS One. 2016;11(5):e0154970. doi: 10.1371/journal.pone.0154970. - DOI - PMC - PubMed
-
- Puig-Barberà J, Natividad-Sancho A, Launay O, Burtseva E, Ciblak MA, Tormos A, et al. 2012-2013 seasonal influenza vaccine effectiveness against influenza hospitalizations: results from the global influenza hospital surveillance network. PLoS One. 2014;9(6):e100497. doi: 10.1371/journal.pone.0100497. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

