Agonists that stimulate secretion promote the recruitment of CFTR into membrane lipid microdomains
- PMID: 31048413
- PMCID: PMC6572005
- DOI: 10.1085/jgp.201812143
Agonists that stimulate secretion promote the recruitment of CFTR into membrane lipid microdomains
Abstract
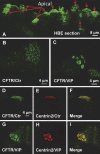
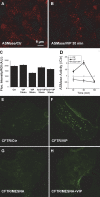
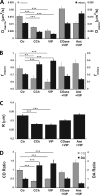
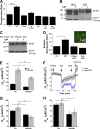
The cystic fibrosis transmembrane conductance regulator (CFTR) is a tightly regulated anion channel that mediates secretion by epithelia and is mutated in the disease cystic fibrosis. CFTR forms macromolecular complexes with many proteins; however, little is known regarding its associations with membrane lipids or the regulation of its distribution and mobility at the cell surface. We report here that secretagogues (agonists that stimulate secretion) such as the peptide hormone vasoactive intestinal peptide (VIP) and muscarinic agonist carbachol increase CFTR aggregation into cholesterol-dependent clusters, reduce CFTR lateral mobility within and between membrane microdomains, and trigger the fusion of clusters into large (3.0 µm2) ceramide-rich platforms. CFTR clusters are closely associated with motile cilia and with the enzyme acid sphingomyelinase (ASMase) that is constitutively bound on the cell surface. Platform induction is prevented by pretreating cells with cholesterol oxidase to disrupt lipid rafts or by exposure to the ASMase functional inhibitor amitriptyline or the membrane-impermeant reducing agent 2-mercaptoethanesulfonate. Platforms are reversible, and their induction does not lead to an increase in apoptosis; however, blocking platform formation does prevent the increase in CFTR surface expression that normally occurs during VIP stimulation. These results demonstrate that CFTR is colocalized with motile cilia and reveal surprisingly robust regulation of CFTR distribution and lateral mobility, most likely through autocrine redox activation of extracellular ASMase. Formation of ceramide-rich platforms containing CFTR enhances transepithelial secretion and likely has other functions related to inflammation and mucosal immunity.
© 2019 Abu-Arish et al.
Figures




Comment in
-
CFTR gets together.J Gen Physiol. 2019 Jun 3;151(6):705. doi: 10.1085/jgp.201912385. Epub 2019 May 10. J Gen Physiol. 2019. PMID: 31076449 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

