Prenatal activity from thalamic neurons governs the emergence of functional cortical maps in mice
- PMID: 31048552
- PMCID: PMC7611000
- DOI: 10.1126/science.aav7617
Prenatal activity from thalamic neurons governs the emergence of functional cortical maps in mice
Abstract
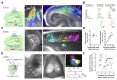
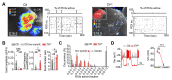
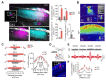
The mammalian brain's somatosensory cortex is a topographic map of the body's sensory experience. In mice, cortical barrels reflect whisker input. We asked whether these cortical structures require sensory input to develop or are driven by intrinsic activity. Thalamocortical columns, connecting the thalamus to the cortex, emerge before sensory input and concur with calcium waves in the embryonic thalamus. We show that the columnar organization of the thalamocortical somatotopic map exists in the mouse embryo before sensory input, thus linking spontaneous embryonic thalamic activity to somatosensory map formation. Without thalamic calcium waves, cortical circuits become hyperexcitable, columnar and barrel organization does not emerge, and the somatosensory map lacks anatomical and functional structure. Thus, a self-organized protomap in the embryonic thalamus drives the functional assembly of murine thalamocortical sensory circuits.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

Comment in
-
Embryonic neural activity wires the brain.Science. 2019 Jun 7;364(6444):933-934. doi: 10.1126/science.aax8048. Science. 2019. PMID: 31171680 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

