A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging
- PMID: 31048701
- PMCID: PMC6497674
- DOI: 10.1038/s41467-019-10119-x
A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging
Abstract
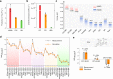
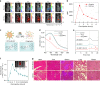
Afterglow imaging with long-lasting luminescence after cessation of light excitation provides opportunities for ultrasensitive molecular imaging; however, the lack of biologically compatible afterglow agents has impeded exploitation in clinical settings. This study presents a generic approach to transforming ordinary optical agents (including fluorescent polymers, dyes, and inorganic semiconductors) into afterglow luminescent nanoparticles (ALNPs). This approach integrates a cascade photoreaction into a single-particle entity, enabling ALNPs to chemically store photoenergy and spontaneously decay it in an energy-relay process. Not only can the afterglow profiles of ALNPs be finetuned to afford emission from visible to near-infrared (NIR) region, but also their intensities can be predicted by a mathematical model. The representative NIR ALNPs permit rapid detection of tumors in living mice with a signal-to-background ratio that is more than three orders of magnitude higher than that of NIR fluorescence. The biodegradability of the ALNPs further heightens their potential for ultrasensitive in vivo imaging.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
Afterglow probes made easy.Nat Methods. 2019 Jul;16(7):577. doi: 10.1038/s41592-019-0483-y. Nat Methods. 2019. PMID: 31249415 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

