Using mobile technology to engage sexual and gender minorities in clinical research
- PMID: 31048870
- PMCID: PMC6497300
- DOI: 10.1371/journal.pone.0216282
Using mobile technology to engage sexual and gender minorities in clinical research
Abstract
Introduction: Historical and current stigmatizing and discriminatory experiences drive sexual and gender minority (SGM) people away from health care and clinical research. Being medically underserved, they face numerous disparities that make them vulnerable to poor health outcomes. Effective methods to engage and recruit SGM people into clinical research studies are needed.
Objectives: To promote health equity and understand SGM health needs, we sought to design an online, national, longitudinal cohort study entitled The PRIDE (Population Research in Identity and Disparities for Equality) Study that enabled SGM people to safely participate, provide demographic and health data, and generate SGM health-related research ideas.
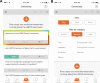
Methods: We developed an iPhone mobile application ("app") to engage and recruit SGM people to The PRIDE Study-Phase 1. Participants completed demographic and health surveys and joined in asynchronous discussions about SGM health-related topics important to them for future study.
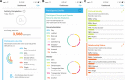
Results: The PRIDE Study-Phase 1 consented 18,099 participants. Of them, 16,394 provided data. More than 98% identified as a sexual minority, and more than 15% identified as a gender minority. The sample was diverse in terms of sexual orientation, gender identity, age, race, ethnicity, geographic location, education, and individual income. Participants completed 24,022 surveys, provided 3,544 health topics important to them, and cast 60,522 votes indicating their opinion of a particular health topic.
Conclusions: We developed an iPhone app that recruited SGM adults and collected demographic and health data for a new national online cohort study. Digital engagement features empowered participants to become committed stakeholders in the research development process. We believe this is the first time that a mobile app has been used to specifically engage and recruit large numbers of an underrepresented population for clinical research. Similar approaches may be successful, convenient, and cost-effective at engaging and recruiting other vulnerable populations into clinical research studies.
Conflict of interest statement
CS and JF are current employees of THREAD Research. AJT is a former employee of THREAD Research. All other authors have no financial or professional conflicts of interest. This project (including all of the authors) did not receive any financial support from Apple, Incorporated. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
-
- Heck NC, Mirabito LA, LeMaire K, Livingston NA, Flentje A. Omitted data in randomized controlled trials for anxiety and depression: A systematic review of the inclusion of sexual orientation and gender identity. Journal of consulting and clinical psychology. 2017;85(1):72–6. Epub 2016/11/16. 10.1037/ccp0000123 PubMed Central PMCID: PMCPMC5161712. - DOI - PMC - PubMed
-
- CDC/National Center for Health Statistics. About the National Health Interview Survey 2019 [cited 2019 April 11]. Available from: https://www.cdc.gov/nchs/nhis/about_nhis.htm.
-
- Thompson JH. Director's Blog: Planned Subjects for the 2020 Census and the American Community Survey: U.S. Census Bureau, U.S. Department of Commerce; 2017. [cited 2018 July 5]. Available from: https://www.census.gov/newsroom/blogs/director/2017/03/planned_subjects_....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

