Elevation of IL-6 and IL-33 Levels in Serum Associated with Lung Fibrosis and Skeletal Muscle Wasting in a Bleomycin-Induced Lung Injury Mouse Model
- PMID: 31049028
- PMCID: PMC6458868
- DOI: 10.1155/2019/7947596
Elevation of IL-6 and IL-33 Levels in Serum Associated with Lung Fibrosis and Skeletal Muscle Wasting in a Bleomycin-Induced Lung Injury Mouse Model
Abstract
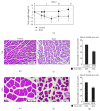
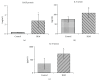
Weight loss due to skeletal muscle atrophy in patients with chronic pulmonary disease is negatively correlated with clinical outcome. Pulmonary fibrosis is a chronic and progressive interstitial lung disease characterized by the dysregulated deposition of the extracellular matrix (ECM) with the destruction of normal tissue, resulting in end-stage organ failure. BLM-induced fibrosis is one of several different experimental models of pulmonary fibrosis, characterized by inflammation and excessive ECM deposition. We directly induced mouse lung injury by the intratracheal administration of bleomycin and monitored the physiological and biochemical changes in lung and skeletal muscle tissues by using lung function testing, ELISA, Western blotting, and immunohistochemistry. Here, we found that BLM-induced lung fibrosis with thickened interstitial lung tissue, including fibronectin and collagen, was correlated with the increased serum concentrations of IL-6 and IL-33 and accompanied by reduced lung function, including FRC (functional residual capacity), C chord (lung compliance), IC (inspiratory capacity), VC (vital capacity), TLC (total lung capacity), and FVC (forced vital capacity) (p < 0.05). The activity of AKT in lung tissue was suppressed, but conversely, the activity of STAT3 was enhanced during lung fibrosis in mice. In addition, we found that the amount of sST2, the soluble form of the IL-33 receptor, was dramatically decreased in lung fibrosis tissues. The skeletal muscle tissue isolated from lung injury mice increased the activation of STAT3 and AMPK, accompanied by an increased amount of Atrogin-1 protein in BLM-induced lung fibrosis mice. The mouse myoblast cell-based model showed that IL-6 and IL-33 specifically activated STAT3 and AMPK signaling, respectively, to induce the expression of the muscle-specific proteolysis markers MuRF1 and Atrogin-1. These data suggested that increased levels of IL-6 and IL-33 in the serum of mice with BLM-induced lung injury may cause lung fibrosis with thickened interstitial lung tissue accompanied by reduced lung function and muscle mass through the activation of STAT3 and AMPK signals.
Figures





References
-
- Raghu G., Collard H. R., Egan J. J., et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American Journal of Respiratory and Critical Care Medicine. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
-
- Raghu G., Depaso W. J., Cain K., et al. Azathioprine combined with prednisone in the treatment of idiopathic pulmonary fibrosis: a prospective double-blind, randomized, placebo-controlled clinical trial. American Review of Respiratory Disease. 1991;144(2):291–296. doi: 10.1164/ajrccm/144.2.291. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

