In Vitro Effect of Estradiol and Progesterone on Ovine Amniotic Epithelial Cells
- PMID: 31049069
- PMCID: PMC6458847
- DOI: 10.1155/2019/8034578
In Vitro Effect of Estradiol and Progesterone on Ovine Amniotic Epithelial Cells
Abstract
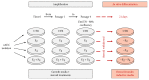
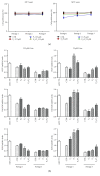
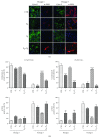
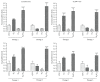
Amniotic epithelial cells (AECs), an emerging source of extrafoetal stem cells, have recently attracted attention for their great regenerative potential. Since AEC amplifications are accompanied by the loss of their native epithelial phenotype and by the progressive reduction of relevant biological properties, the issue to be addressed is the development of effective culture protocols. In this context, recently, it has been demonstrated that progesterone (P4) supplementation during ovine AEC (oAEC) expansion could prevent the undesirable epithelial-mesenchymal transition (EMT). In contrast, there is no information to date on the role of the other pregnancy steroids in culture. With this aim, the present study has been designed to clarify the impact of estradiol (E2), alone or in combination with P4 (12.5 μM and 25 μM), during oAEC amplification. Steroid supplementations were assessed by testing oAEC proliferation, stemness, EMT, and osteogenic or chondrogenic plasticity. The results indicated that EMT can be prevented exclusively in the presence of high doses of P4, while it occurred rapidly in cells exposed to E2 as denoted by protein (cytokeratin-8 and alpha-SMA) and gene expression (vimentin and snail) profiles. Moreover, steroid exposure was able to influence highly oAEC plasticity. Particularly, P4-treated cells displayed a precommitment towards osteogenic lineage, confirmed by the upregulation of OCN, RUNX2, and the greater deposition of calcium nodules. Conversely, P4 exposure inhibited oAEC chondrogenic differentiation, which was induced in E2-treated cells as confirmed by the upregulation of chondrogenesis-related genes (SOX9, ACAN, and COL2A1) and by the accumulation of Alcian blue-positive extracellular matrix. Simultaneously, E2-treated cells remained unresponsive to osteogenic inductive stimuli. In conclusion, media supplementation with high doses of steroids may be adopted to modulate phenotype and plasticity during oAEC amplification. Relevantly, the osteo or chondro steroid-induced precommitment may open unprecedented cell-based therapies to face the unsolved orthopaedic issues related to osteochondral regeneration.
Figures

Similar articles
-
Progesterone prevents epithelial-mesenchymal transition of ovine amniotic epithelial cells and enhances their immunomodulatory properties.Sci Rep. 2017 Jun 19;7(1):3761. doi: 10.1038/s41598-017-03908-1. Sci Rep. 2017. PMID: 28630448 Free PMC article.
-
Amniotic Epithelial Cell Culture.Methods Mol Biol. 2018;1817:67-78. doi: 10.1007/978-1-4939-8600-2_7. Methods Mol Biol. 2018. PMID: 29959703
-
Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation.PLoS One. 2013 May 17;8(5):e63256. doi: 10.1371/journal.pone.0063256. Print 2013. PLoS One. 2013. PMID: 23696804 Free PMC article.
-
Amniotic Membrane and Amniotic Epithelial Cell Culture.Methods Mol Biol. 2024;2749:135-149. doi: 10.1007/978-1-0716-3609-1_13. Methods Mol Biol. 2024. PMID: 38133781
-
The expression of genes encoding zona pellucida glycoproteins in canine cumulus-oocyte complexes cultured in vitro in media supplemented with progesterone and estradiol.Theriogenology. 2012 Feb;77(3):684-93. doi: 10.1016/j.theriogenology.2011.09.008. Epub 2011 Nov 23. Theriogenology. 2012. PMID: 22115812
Cited by
-
Characterization of KLHL14 anti-oncogenic action in malignant mesothelioma.Heliyon. 2024 Mar 9;10(6):e27731. doi: 10.1016/j.heliyon.2024.e27731. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38509883 Free PMC article.
-
Hypoxia-Mimetic CoCl2 Agent Enhances Pro-Angiogenic Activities in Ovine Amniotic Epithelial Cells-Derived Conditioned Medium.Cells. 2022 Jan 28;11(3):461. doi: 10.3390/cells11030461. Cells. 2022. PMID: 35159271 Free PMC article.
-
Adipose Tissue- and Bone Marrow-Derived Mesenchymal Stem Cells from Sheep: Culture Characteristics.Animals (Basel). 2021 Jul 21;11(8):2153. doi: 10.3390/ani11082153. Animals (Basel). 2021. PMID: 34438611 Free PMC article.
-
"In medio stat virtus": Insights into hybrid E/M phenotype attitudes.Front Cell Dev Biol. 2022 Nov 18;10:1038841. doi: 10.3389/fcell.2022.1038841. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36467417 Free PMC article. Review.
-
Mechanobiological Strategies to Enhance Ovine (Ovis aries) Adipose-Derived Stem Cells Tendon Plasticity for Regenerative Medicine and Tissue Engineering Applications.Animals (Basel). 2024 Jul 31;14(15):2233. doi: 10.3390/ani14152233. Animals (Basel). 2024. PMID: 39123758 Free PMC article.
References
-
- Malek A., Bersinger N. A. Human placental stem cells: biomedical potential and clinical relevance. Journal of Stem Cells. 2011;6(2):75–92. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

