Metal Recovery from Spent Samarium-Cobalt Magnets Using a Trichloride Ionic Liquid
- PMID: 31049272
- PMCID: PMC6488128
- DOI: 10.1021/acssuschemeng.8b05604
Metal Recovery from Spent Samarium-Cobalt Magnets Using a Trichloride Ionic Liquid
Abstract
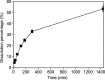
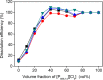
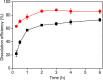
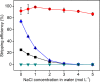
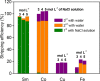
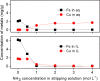
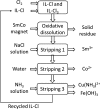
Recycling of samarium-cobalt (SmCo) magnets is essential due to the limited resources of the mentioned metals and their high economic importance. The ionic liquid (IL) trihexyltetradecylphosphonium trichloride, [P666,14][Cl3], which can safely store chlorine gas in the form of the trichloride anion, was used as an oxidizing solvent for the recovery of metals from spent SmCo magnets. The dissolution was studied considering various mixtures of the ILs [P666,14][Cl3] and [P666,14]Cl, solid-to-liquid ratios and different temperatures. The results showed that the maximum capacity of [P666,14][Cl3] for SmCo magnets was 71 ± 1 mg/g of [P666,14][Cl3], in the presence of an extra source of coordinating chloride ions. The maximum loading of the IL could be reached within 3 h at 50 °C. Four stripping steps effectively removed all metals from the loaded IL, where sodium chloride solution (3 mol L-1), twice water and ammonia solution (3 mol L-1) were used consecutively as the stripping solvents. The regenerated IL showed a similar dissolution performance as fresh IL. Oxidative dissolution of metals in trichloride ILs is easily transferable to the recycling of valuable metals from other end-of-life products such as neodymium-iron-boron magnets and nickel metal hydride batteries.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Strnat K. J.; Strnat R. M. W. Rare-earth cobalt permanent-magnets. J. Magn. Magn. Mater. 1991, 100 (1–3), 38–56. 10.1016/0304-8853(91)90811-N. - DOI
-
- Buschow K. H. J. Intermetallic compounds of rare-earth and 3d transition-metals. Rep. Prog. Phys. 1977, 40 (10), 1179–1256. 10.1088/0034-4885/40/10/002. - DOI
-
- Binnemans K.; Jones P. T.; Blanpain B.; Van Gerven T.; Yang Y.; Walton A.; Buchert M. Recycling of rare earths: a critical review. J. Cleaner Prod. 2013, 51, 1–22. 10.1016/j.jclepro.2012.12.037. - DOI
-
- Binnemans K.; Jones P. T. Rare Earths and the Balance Problem. J. Sustain. Metall. 2015, 1 (1), 29–38. 10.1007/s40831-014-0005-1. - DOI
-
- Eldosouky A.; Škulj I. Recycling of SmCo5 magnets by HD process. J. Magn. Magn. Mater. 2018, 454, 249–253. 10.1016/j.jmmm.2018.01.064. - DOI
LinkOut - more resources
Full Text Sources
