p63 establishes epithelial enhancers at critical craniofacial development genes
- PMID: 31049400
- PMCID: PMC6494499
- DOI: 10.1126/sciadv.aaw0946
p63 establishes epithelial enhancers at critical craniofacial development genes
Abstract
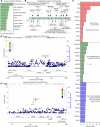
The transcription factor p63 is a key mediator of epidermal development. Point mutations in p63 in patients lead to developmental defects, including orofacial clefting. To date, knowledge on how pivotal the role of p63 is in human craniofacial development is limited. Using an inducible transdifferentiation model, combined with epigenomic sequencing and multicohort meta-analysis of genome-wide association studies data, we show that p63 establishes enhancers at craniofacial development genes to modulate their transcription. Disease-specific substitution mutation in the DNA binding domain or sterile alpha motif protein interaction domain of p63, respectively, eliminates or reduces establishment of these enhancers. We show that enhancers established by p63 are highly enriched for single-nucleotide polymorphisms associated with nonsyndromic cleft lip ± cleft palate (CL/P). These orthogonal approaches indicate a strong molecular link between p63 enhancer function and CL/P, illuminating molecular mechanisms underlying this developmental defect and revealing vital regulatory elements and new candidate causative genes.
Figures




References
-
- Yang A., Kaghad M., Wang Y., Gillett E., Fleming M. D., Dötsch V., Andrews N. C., Caput D., McKeon F., p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 (1998). - PubMed
-
- Yang A., Schweitzer R., Sun D., Kaghad M., Walker N., Bronson R. T., Tabin C., Sharpe A., Caput D., Crum C., McKeon F., p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 (1999). - PubMed
-
- Mills A. A., Zheng B., Wang X. J., Vogel H., Roop D. R., Bradley A., p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 (1999). - PubMed
-
- Celli J., Duijf P., Hamel B. C. J., Bamshad M., Kramer B., Smits A. P. T., Newbury-Ecob R., Hennekam R. C. M., van Buggenhout G., van Haeringen A., Woods C. G., van Essen A. J., de Waal R., Vriend G., Haber D. A., Yang A., McKeon F., Brunner H. G., van Bokhoven H., Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell 99, 143–153 (1999). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

