The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV
- PMID: 31049758
- PMCID: PMC6545178
- DOI: 10.1007/s10147-019-01431-z
The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV
Abstract
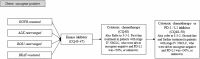
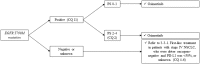
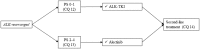
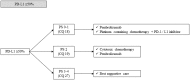
According to rapid development of chemotherapy in advanced non-small cell lung cancer (NSCLC), the Japan Lung Cancer Society has been updated its own guideline annually since 2010. In this latest version, all of the procedure was carried out in accordance with grading of recommendations assessment, development and evaluation (GRADE) system. It includes comprehensive literature search, systematic review, and determination of the recommendation by multidisciplinary expert panel which consisted of medical doctors, pharmacists, nurses, statisticians, and patients from patient advocacy group. Recently, we have had various types of chemotherapeutic drugs like kinase inhibitors or immune-checkpoint inhibitors. Thus, the guideline proposes to categorize patients into three entities: (1) driver oncogene-positive, (2) PD-L1 ≥ 50%, and (3) others. Based on this subgroup, 31 clinical questions were described. We believe that this attempt enables clinicians to choose appropriate treatment easier. Here, we report an English version of the Japan Lung Cancer Society Guidelines 2018 for NSCLC, stages IV.
Keywords: Chemotherapy; Guideline; Kinase inhibitor; Non-small cell lung cancer; Programed cell death-1 inhibitor; Programed death-ligand 1 inhibitor.
Conflict of interest statement
Hiroaki Akamatsu received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Eli Lilly, MSD, Ono Pharmaceutical Co. Ltd, Pfizer, and Taiho Pharmaceutical Co. Ltd. He received research funding from MSD. Kiichiro Ninomiya received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, and MSD. Hirotsugu Kenmotsu received honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd, Ono Pharmaceutical Co. Ltd. Boeringer Ingelheim, Eli Lilly K.K., Kyowa Hakko Kirin Co. Ltd., Bristol-Myers Squibb, MSD, and Novartis Pharma K.K. He also received research funding from AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd, and Boeringer Ingelheim. Masahiro Morise received honoraria from AstraZeneca, Chugai Pharmaceutical Co. Ltd, Eli Lilly, MSD, Ono Pharmaceutical Co. Ltd, and Pfizer. He also received research funding from AstraZeneca K.K., Boehringer Ingelheim, Chugai Pharmaceutical Co. Ltd, Pfizer, Ono Pharmaceutical Co. Ltd., Merck Serono, Kissei, Novartis and Taiho Pharmaceutical Co. Ltd. Haruko Daga received honoraria from Boehringer Ingelheim, Chugai Pharmaceutical Co. Ltd, and MSD. Yasushi Goto received honoraria from AstraZeneca, Eli Lilly, Chugai, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical Co. Ltd., Bristol Myers Squibb, Pfizer, MSD, Shionogi Pharma and Novartis. He also received research funding from Abbvie, Eli Lilly, Taiho Pharmaceutical, Bristol Myers Squibb, and Ono Pharmaceutical Co. Ltd. Toshiyuki Kozuki received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Eli Lilly, Kyowa Hakko Kirin Co, MSD, Nippon Kayaku, Ono Pharmaceutical Co. Ltd, Pfizer and Taiho Pharmaceutical Co. Ltd. He received research funding from AstraZeneca Chugai Pharmaceutical Co. Ltd, Eli Lilly and Merck Serono. Satoru Miura received honoraria from AstraZeneca, Boehringer Ingelheim, Chugai Pharmaceutical Co. Ltd, Eli Lilly and MSD. Takaaki Sasaki received honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo Co. Ltd., Eli Lilly and Novartis. He received research funding from Boehringer Ingelheim and Pfizer. Akihiro Tamiya received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Ono Pharmaceutical Co. Ltd. and Eli Lilly. He received research funding from AstraZeneca, Bristol-Myers Squibb and Ono Pharmaceutical Co. Ltd. Yukari Tsubata received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo Co. Ltd. and Kyowa Hakko Kirin. She received research funding from Daiichi Sankyo Co. Ltd. and Ono Pharmaceutical Co. Ltd. Hiroshige Yoshioka received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Eli Lilly, MSD, Novartis, Ono Pharmaceutical Co. Ltd., Pfizer, and Taiho Pharmaceutical Co. Ltd. Yoshihiro Hattori received honoraria from AstraZeneca, Boehringer Ingelheim, Chugai Pharmaceutical Co. Ltd, Eli Lilly, MSD, Novartis, Ono Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd. He received research funding from MSD and Ono Pharmaceutical Co. Ltd. Hidenori Mizugaki received honoraria from AstraZeneca, Boehringer Ingelheim, and Chugai Pharmaceutical Co. Ltd. He received research funding from Boehringer Ingelheim. Kaname Nosaki received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Eli Lilly, Kyowa Hakko Kirin, MSD, Nippon Kayaku, Novartis, Ono Pharmaceutical Co. Ltd., Pfizer, and Taiho Pharmaceutical Co. Ltd. He received research funding from MSD and Novartis. Yusuke Okuma received honoraria from Boehringer Ingelheim, AstraZeneca, MSD, Novartis, Taiho Pharmaceutical Co. Ltd, and Eli-Lilly. He received research funding from Chugai Pharmaceutical Co. Ltd and Takeda Pharmaceutical Co. Ltd. Kentaro Tanaka received honoraria from Chugai Pharmaceutical Co. Ltd. Shigeki Umemura received research funding from MSD. Takeharu Yamanaka received honoraria from Boehringer Ingelheim, Chugai Pharmaceutical Co. Ltd, Taiho Pharmaceutical Co. Ltd, and Takeda Pharmaceutical Co. Ltd. He received research funding from Taiho Pharmaceutical Co. Ltd and Takeda Pharmaceutical Co. Ltd. Satoshi Morita received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Eli Lilly, MSD, Ono Pharmaceutical Co. Ltd., Pfizer, and Taiho Pharmaceutical Co. Ltd. He received research funding from Boehringer Ingelheim. Makoto Maemondo received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Eli Lilly, MSD, Novartis, Ono Pharmaceutical Co. Ltd., Pfizer, and Taiho Pharmaceutical Co. Ltd. He received research funding from Boehringer Ingelheim. Takashi Seto received honoraria from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Eli Lilly, Kissei Pharmaceutical Co. Ltd, MSD, Novartis, Ono Pharmaceutical Co. Ltd., Pfizer, Roche Singapore, Takeda Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Thermo Fischer Scientific and Yakult Honsha Co. Ltd. He received research funding from Astellas Pharma, AstraZeneca, Bayer Yakuhin, Boehringer Ingelheim, Chugai Pharmaceutical Co. Ltd, Daiichi Sankyo, Eisai, Eli Lilly, Kissei Pharmaceutical Co. Ltd, LOXO Oncology, Merck Serono, MSD, Novartis, Pfizer and Takeda Pharmaceutical Co. Ltd. Nobuyuki Yamamoto received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Daiichi Sankyo, Eli Lilly, MSD, Novartis, Ono Pharmaceutical Co. Ltd., Pfizer, Taiho Pharmaceutical Co. Ltd. and Yakult Honsha Co. Ltd. He received research funding from Boehringer Ingelheim, Chugai Pharmaceutical Co. Ltd, Eli Lilly, and MSD. Others are no conflicts of interest.
Figures








References
-
- Baggstrom MQ, Stinchcombe TE, Fried DB, et al. Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: a meta-analysis. J Thorac Oncol. 2007;2(9):845–853. - PubMed
-
- Fujiwara Y, Hotta K, Di Maio M, et al. Time trend in treatment-related deaths of patients with advanced non-small-cell lung cancer enrolled into phase III trials of systemic treatment. Ann Oncol. 2011;22(2):376–382. - PubMed
-
- Anderson H, Hopwood P, Stephens RJ, et al. Gemcitabine plus best supportive care(BSC)vs BSC in inoperable non-small cell lung cancer—a randomized trial with quality of life as the primary outcome. UK NSCLC Gemcitabine Group. Non-Small Cell Lung Cancer. Br J Cancer. 2000;83(4):447–453. - PMC - PubMed
-
- Sederholm C, Hillerdal G, Lamberg K, et al. Phase III trial of gemcitabine plus carboplatin versus single-agent gemcitabine in the treatment of locally advanced or metastatic non-small-cell lung cancer: the Swedish Lung Cancer Study Group. J Clin Oncol. 2005;23(33):8380–8388. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

