GATA-1 isoforms differently contribute to the production and compartmentation of reactive oxygen species in the myeloid leukemia cell line K562
- PMID: 31049966
- PMCID: PMC6767011
- DOI: 10.1002/jcp.28688
GATA-1 isoforms differently contribute to the production and compartmentation of reactive oxygen species in the myeloid leukemia cell line K562
Abstract
Maintenance of a balanced expression of the two isoforms of the transcription factor GATA-1, the full-length protein (GATA-1FL ) and a shorter isoform (GATA-1 S ), contributes to control hematopoiesis, whereas their dysregulation can alter the differentiation/proliferation potential of hematopoietic precursors thereby eventually leading to a variety of hematopoietic disorders. Although it is well established that these isoforms play opposite roles in these remarkable processes, most of the molecular pathways involved remain unknown. Here, we demonstrate that GATA-1FL and GATA-1S are able to differently influence intracellular redox states and reactive oxygen species (ROS) compartmentation in the erythroleukemic K562 cell line, thus shedding novel mechanistic insights into the processes of cell proliferation and apoptosis resistance in myeloid precursors. Furthermore, given the role played by ROS signaling as a strategy to escape apoptosis and evade cell-mediated immunity in myeloid cells, this study highlights a mechanism through which aberrant expression of GATA-1 isoforms could play a role in the leukemogenic process.
Keywords: GATA-1; mitochondria remodeling; myeloid leukemia; oxidative stress; succinate dehydrogenase subunit C (SDHC).
© 2019 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Figures
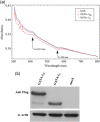

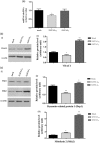
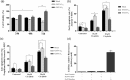
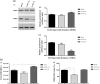



Similar articles
-
Exploring the Leukemogenic Potential of GATA-1S, the Shorter Isoform of GATA-1: Novel Insights into Mechanisms Hampering Respiratory Chain Complex II Activity and Limiting Oxidative Phosphorylation Efficiency.Antioxidants (Basel). 2021 Oct 12;10(10):1603. doi: 10.3390/antiox10101603. Antioxidants (Basel). 2021. PMID: 34679737 Free PMC article.
-
Over-Expressed GATA-1S, the Short Isoform of the Hematopoietic Transcriptional Factor GATA-1, Inhibits Ferroptosis in K562 Myeloid Leukemia Cells by Preventing Lipid Peroxidation.Antioxidants (Basel). 2023 Feb 21;12(3):537. doi: 10.3390/antiox12030537. Antioxidants (Basel). 2023. PMID: 36978786 Free PMC article.
-
Do reactive oxygen species play a role in myeloid leukemias?Blood. 2011 Jun 2;117(22):5816-26. doi: 10.1182/blood-2011-01-326025. Epub 2011 Mar 11. Blood. 2011. PMID: 21398578 Review.
-
miR-1202 acts as anti-oncomiR in myeloid leukaemia by down-modulating GATA-1S expression.Open Biol. 2024 Feb;14(2):230319. doi: 10.1098/rsob.230319. Epub 2024 Feb 14. Open Biol. 2024. PMID: 38350611 Free PMC article.
-
Model animals for the study of oxidative stress from complex II.Biochim Biophys Acta. 2013 May;1827(5):588-97. doi: 10.1016/j.bbabio.2012.10.016. Epub 2012 Nov 6. Biochim Biophys Acta. 2013. PMID: 23142169 Review.
Cited by
-
Combined Effects of Tanshinone IIA and an Autophagy Inhibitor on the Apoptosis of Leukemia Cells via p53, Apoptosis-Related Proteins and Oxidative Stress Pathways.Integr Cancer Ther. 2022 Jan-Dec;21:15347354221117776. doi: 10.1177/15347354221117776. Integr Cancer Ther. 2022. PMID: 35996358 Free PMC article.
-
Tetrahydrobenzimidazole TMQ0153 targets OPA1 and restores drug sensitivity in AML via ROS-induced mitochondrial metabolic reprogramming.J Exp Clin Cancer Res. 2025 Apr 7;44(1):114. doi: 10.1186/s13046-025-03372-0. J Exp Clin Cancer Res. 2025. PMID: 40197337 Free PMC article.
-
Effect of Different Coffee Brews on Tryptophan Metabolite-Induced Cytotoxicity in HT-29 Human Colon Cancer Cells.Antioxidants (Basel). 2022 Dec 14;11(12):2458. doi: 10.3390/antiox11122458. Antioxidants (Basel). 2022. PMID: 36552667 Free PMC article.
-
Antioxidant Systems as Modulators of Ferroptosis: Focus on Transcription Factors.Antioxidants (Basel). 2024 Feb 28;13(3):298. doi: 10.3390/antiox13030298. Antioxidants (Basel). 2024. PMID: 38539832 Free PMC article. Review.
-
Case report: A safeguard in the sea of variants of uncertain significance: a case study on child with high risk neuroblastoma and acute myeloid leukemia.Front Oncol. 2024 Jan 8;13:1324013. doi: 10.3389/fonc.2023.1324013. eCollection 2023. Front Oncol. 2024. PMID: 38260858 Free PMC article.
References
-
- Bellisola, G. , & Sorio, C. (2012). Infrared spectroscopy, and microscopy in cancer research and diagnosis. American Journal of Cancer Research, 2, 1–21. www.ajcr.us - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

