The Glutamine Transporter Slc38a1 Regulates GABAergic Neurotransmission and Synaptic Plasticity
- PMID: 31050701
- PMCID: PMC6918930
- DOI: 10.1093/cercor/bhz055
The Glutamine Transporter Slc38a1 Regulates GABAergic Neurotransmission and Synaptic Plasticity
Abstract
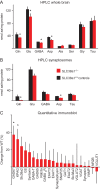
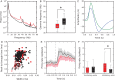
GABA signaling sustains fundamental brain functions, from nervous system development to the synchronization of population activity and synaptic plasticity. Despite these pivotal features, molecular determinants underscoring the rapid and cell-autonomous replenishment of the vesicular neurotransmitter GABA and its impact on synaptic plasticity remain elusive. Here, we show that genetic disruption of the glutamine transporter Slc38a1 in mice hampers GABA synthesis, modifies synaptic vesicle morphology in GABAergic presynapses and impairs critical period plasticity. We demonstrate that Slc38a1-mediated glutamine transport regulates vesicular GABA content, induces high-frequency membrane oscillations and shapes cortical processing and plasticity. Taken together, this work shows that Slc38a1 is not merely a transporter accumulating glutamine for metabolic purposes, but a key component regulating several neuronal functions.
Keywords: GABA; SAT1; SNAT1; Slc38; neurotransmitter replenishment.
© The Author(s) 2019. Published by Oxford University Press.
Figures





References
-
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. 2004. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 92(1):600–608. - PubMed
-
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. 2007. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 87(4):1215–1284. - PubMed
-
- Berghuis P, Dobszay MB, Sousa KM, Schulte G, Mager PP, Hartig W, Gorcs TJ, Zilberter Y, Ernfors P, Harkany T. 2004. Brain-derived neurotrophic factor controls functional differentiation and microcircuit formation of selectively isolated fast-spiking GABAergic interneurons. Eur J Neurosci. 20(5):1290–1306. - PubMed
-
- Bogen IL, Boulland JL, Mariussen E, Wright MS, Fonnum F, Kao HT, Walaas SI. 2006. Absence of synapsin I and II is accompanied by decreases in vesicular transport of specific neurotransmitters. J Neurochem. 96(5):1458–1466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

