Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up
- PMID: 31050707
- PMCID: PMC6594457
- DOI: 10.1093/annonc/mdz127
Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up
Abstract
Background: Novel second-line treatments are needed for patients with advanced urothelial cancer (UC). Interim analysis of the phase III KEYNOTE-045 study showed a superior overall survival (OS) benefit of pembrolizumab, a programmed death 1 inhibitor, versus chemotherapy in patients with advanced UC that progressed on platinum-based chemotherapy. Here we report the long-term safety and efficacy outcomes of KEYNOTE-045.
Patients and methods: Adult patients with histologically/cytologically confirmed UC whose disease progressed after first-line, platinum-containing chemotherapy were enrolled. Patients were randomly assigned 1 : 1 to receive pembrolizumab [200 mg every 3 weeks (Q3W)] or investigator's choice of paclitaxel (175 mg/m2 Q3W), docetaxel (75 mg/m2 Q3W), or vinflunine (320 mg/m2 Q3W). Primary end points were OS and progression-free survival (PFS) per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) by blinded independent central radiology review (BICR). A key secondary end point was objective response rate per RECIST v1.1 by BICR.
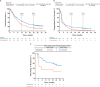
Results: A total of 542 patients were enrolled (pembrolizumab, n = 270; chemotherapy, n = 272). Median follow-up as of 26 October 2017 was 27.7 months. Median 1- and 2-year OS rates were higher with pembrolizumab (44.2% and 26.9%, respectively) than chemotherapy (29.8% and 14.3%, respectively). PFS rates did not differ between treatment arms; however, 1- and 2-year PFS rates were higher with pembrolizumab. The objective response rate was also higher with pembrolizumab (21.1% versus 11.0%). Median duration of response to pembrolizumab was not reached (range 1.6+ to 30.0+ months) versus chemotherapy (4.4 months; range 1.4+ to 29.9+ months). Pembrolizumab had lower rates of any grade (62.0% versus 90.6%) and grade ≥3 (16.5% versus 50.2%) treatment-related adverse events than chemotherapy.
Conclusions: Long-term results (>2 years' follow-up) were consistent with those of previously reported analyses, demonstrating continued clinical benefit of pembrolizumab over chemotherapy for efficacy and safety for treatment of locally advanced/metastatic, platinum-refractory UC.
Trial registration: ClinicalTrials.gov: NCT02256436.
Keywords: PD-1; PD-L1; pembrolizumab; urothelial cancer.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
Comment in
-
Commentary: 2-year follow up of pembrolizumab as second-line therapy for advanced urothelial cancer ("KEYNOTE 045").Transl Androl Urol. 2019 Oct;8(5):409-413. doi: 10.21037/tau.2019.09.19. Transl Androl Urol. 2019. PMID: 31807417 Free PMC article. No abstract available.
-
Second line immune checkpoint inhibition in urothelial cancer.Transl Androl Urol. 2019 Oct;8(5):414-420. doi: 10.21037/tau.2019.09.18. Transl Androl Urol. 2019. PMID: 31807418 Free PMC article. No abstract available.
References
-
- Seiwert TY, Burtness B, Mehra R. et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016; 17(7): 956–965. - PubMed
-
- Plimack ER, Bellmunt J, Gupta S. et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 2017; 18(2): 212–220. - PubMed
-
- KEYTRUDA®(Pembrolizumab) for Injection, for Intravenous Use [prescribing information]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2019.
-
- Vaughn DJ, Bellmunt J, Fradet Y. et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. JCO 2018; 36(16): 1579–1587. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

