Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs
- PMID: 31050774
- PMCID: PMC6503493
- DOI: 10.1001/jamanetworkopen.2019.2535
Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs
Abstract
Importance: Immunotherapy checkpoint inhibitors have generated considerable interest because of durable responses in a number of hitherto intractable tumor types.
Objective: To estimate the percentage of patients with cancer in the United States who are eligible for and respond to checkpoint inhibitor drugs approved for oncology indications by the US Food and Drug Administration (FDA).
Design, setting, and participants: Retrospective cross-sectional study performed from June 2018 through October 2018 using publicly available data to determine (1) demographic characteristics of patients with advanced or metastatic cancer, (2) FDA data on checkpoint inhibitors approved from January 2011 through August 2018, (3) measures of response from drug labels, and (4) published reports estimating the frequency of various inclusion criteria.
Main outcomes and measures: The estimated percentages of US patients with cancer who are eligible for and who respond to immunotherapy checkpoint inhibitor drugs, by year.
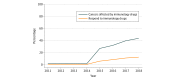
Results: Six checkpoint inhibitor drugs were approved for 14 indications between March 25, 2011, and August 17, 2018. The estimated percentage of patients with cancer who were eligible for checkpoint inhibitor drugs increased from 1.54% (95% CI, 1.51%-1.57%) in 2011 to 43.63% (95% CI, 43.51%-43.75%) in 2018. The percentage of patients with cancer estimated to respond to checkpoint inhibitor drugs was 0.14% (95% CI, 0.13%-0.15%) in 2011 when ipilimumab was approved for unresectable or metastatic melanoma and increased to 5.86% (95% CI, 5.80%-5.92%) by 2015. By 2018, the estimated percentage of responders increased to 12.46% (95% CI, 12.37%-12.54%).
Conclusions and relevance: The estimated percentages of patients who are eligible for and who respond to checkpoint inhibitor drugs are higher than reported estimates for drugs approved for genome-driven oncology but remain modest. Future research should explore biomarkers to maximize the benefit of immunotherapy among patients receiving it.
Conflict of interest statement
Figures
Comment in
-
Keeping Checkpoint Inhibitors in Check.JAMA Netw Open. 2019 May 3;2(5):e192546. doi: 10.1001/jamanetworkopen.2019.2546. JAMA Netw Open. 2019. PMID: 31050772 No abstract available.
References
-
- The Nobel Prize in Physiology or Medicine 2018 [press release]. Stockholm, Sweden: The Nobel Assembly at Karolinska Institutet; October 1, 2018.
-
- Rodriguez Fernandez C. Are PD-1 and PD-L1 checkpoint inhibitors as good as we thought? https://labiotech.eu/features/pd-1-pd-l1-checkpoint-inhibitors/. Published September 4, 2018. Accessed October 12, 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

