Cardioprotection of PLGA/gelatine cardiac patches functionalised with adenosine in a large animal model of ischaemia and reperfusion injury: A feasibility study
- PMID: 31050859
- PMCID: PMC6771506
- DOI: 10.1002/term.2875
Cardioprotection of PLGA/gelatine cardiac patches functionalised with adenosine in a large animal model of ischaemia and reperfusion injury: A feasibility study
Abstract
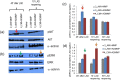
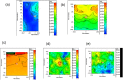
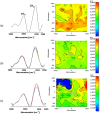
The protection from ischaemia-reperfusion-associated myocardial infarction worsening remains a big challenge. We produced a bioartificial 3D cardiac patch with cardioinductive properties on stem cells. Its multilayer structure was functionalised with clinically relevant doses of adenosine. We report here the first study on the potential of these cardiac patches in the controlled delivery of adenosine into the in vivo ischaemic-reperfused pig heart. A Fourier transform infrared chemical imaging approach allowed us to perform a characterisation, complementary to the histological and biochemical analyses on myocardial samples after in vivo patch implantation, increasing the number of investigations and results on the restricted number of pigs (n = 4) employed in this feasibility step. In vitro tests suggested that adenosine was completely released by a functionalised patch, a data that was confirmed in vivo after 24 hr from patch implantation. Moreover, the adenosine-loaded patch enabled a targeted delivery of the drug to the ischaemic-reperfused area of the heart, as highlighted by the activation of the pro-survival signalling reperfusion injury salvage kinases pathway. At 3 months, though limited to one animal, the used methods provided a picture of a tissue in dynamic conditions, associated to the biosynthesis of new collagen and to a non-fibrotic outcome of the healing process underway. The synergistic effect between the functionalised 3D cardiac patch and adenosine cardioprotection might represent a promising innovation in the treatment of reperfusion injury. As this is a feasibility study, the clinical implications of our findings will require further in vivo investigation on larger numbers of ischaemic-reperfused pig hearts.
Keywords: FT-IR spectroscopy; RISK pathway; adenosine; cardiac patch; cardioprotection; large-animal model.
© 2019 The Authors Journal of Tissue Engineering and Regenerative Medicine Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Cristallini, C. , Cibrario Rocchietti, E. , Accomasso, L. , Folino, A. , Gallina, C. , Muratori, L. , … Giachino, C. (2014). The effect of bioartificial constructs that mimic myocardial structure and biomechanical properties on stem cell commitment towards cardiac lineage. Biomaterials, 35, 92–104. 10.1016/j.biomaterials.2013.09.058 - DOI - PubMed
-
- Cristallini, C. , Cibrario Rocchietti, E. , Gagliardi, M. , Mortati, L. , Saviozzi, S. , Bellotti, E. , … Giachino, C. (2016). Micro and macro‐structured PLGA/gelatin scaffolds promote early cardiogenic commitment of human mesenchymal stem cells in vitro. Stem Cells International, 2016:7176154, 1–16. 10.1155/2016/7176154 - DOI - PMC - PubMed
-
- Donato, M. , D'Annunzio, V. , Berg, G. , Gonzalez, G. , Schreier, L. , Morales, C. , … Gelpi, R. J. (2007). Ischemic postconditioning reduces infarct size by activation of A1 receptors and K+(ATP) channels in both normal and hypercholesterolemic rabbits. Journal of Cardiovascular Pharmacology, 49, 287–292. 10.1097/FJC.0b013e31803c55fe - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

