Rare Variants in BNC2 Are Implicated in Autosomal-Dominant Congenital Lower Urinary-Tract Obstruction
- PMID: 31051115
- PMCID: PMC6506863
- DOI: 10.1016/j.ajhg.2019.03.023
Rare Variants in BNC2 Are Implicated in Autosomal-Dominant Congenital Lower Urinary-Tract Obstruction
Abstract
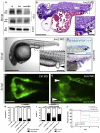
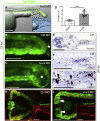
Congenital lower urinary-tract obstruction (LUTO) is caused by anatomical blockage of the bladder outflow tract or by functional impairment of urinary voiding. About three out of 10,000 pregnancies are affected. Although several monogenic causes of functional obstruction have been defined, it is unknown whether congenital LUTO caused by anatomical blockage has a monogenic cause. Exome sequencing in a family with four affected individuals with anatomical blockage of the urethra identified a rare nonsense variant (c.2557C>T [p.Arg853∗]) in BNC2, encoding basonuclin 2, tracking with LUTO over three generations. Re-sequencing BNC2 in 697 individuals with LUTO revealed three further independent missense variants in three unrelated families. In human and mouse embryogenesis, basonuclin 2 was detected in lower urinary-tract rudiments. In zebrafish embryos, bnc2 was expressed in the pronephric duct and cloaca, analogs of the mammalian lower urinary tract. Experimental knockdown of Bnc2 in zebrafish caused pronephric-outlet obstruction and cloacal dilatation, phenocopying human congenital LUTO. Collectively, these results support the conclusion that variants in BNC2 are strongly implicated in LUTO etiology as a result of anatomical blockage.
Keywords: BNC2; LUTO; basonuclin 2; cloacae; distal pronephric outlet obstruction; functional genetics; lower urinary tract obstruction; posterior urethral valve; pronephric development; zebrafish.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Expanding congenital abnormalities of the kidney and urinary tract (CAKUT) genetics: basonuclin 2 (BNC2) and lower urinary tract obstruction.Ann Transl Med. 2019 Sep;7(Suppl 6):S226. doi: 10.21037/atm.2019.08.73. Ann Transl Med. 2019. PMID: 31656805 Free PMC article. No abstract available.
References
-
- Malin G., Tonks A.M., Morris R.K., Gardosi J., Kilby M.D. Congenital lower urinary tract obstruction: A population-based epidemiological study. BJOG. 2012;119:1455–1464. - PubMed
-
- Gauthier J., Ouled Amar Bencheikh B., Hamdan F.F., Harrison S.M., Baker L.A., Couture F., Thiffault I., Ouazzani R., Samuels M.E., Mitchell G.A. A homozygous loss-of-function variant in MYH11 in a case with megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur. J. Hum. Genet. 2015;23:1266–1268. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

