Using metabolic profiling and gene expression analyses to explore molecular effects of replacing saturated fat with polyunsaturated fat-a randomized controlled dietary intervention study
- PMID: 31051508
- PMCID: PMC6499508
- DOI: 10.1093/ajcn/nqy356
Using metabolic profiling and gene expression analyses to explore molecular effects of replacing saturated fat with polyunsaturated fat-a randomized controlled dietary intervention study
Erratum in
-
Corrigendum to "Using metabolic profiling and gene expression analyses to explore molecular effects of replacing saturated fat with polyunsaturated fat-a randomized controlled dietary intervention study" Am J Clin Nutr 2019;109:247-48.Am J Clin Nutr. 2020 Jan 1;111(1):232-236. doi: 10.1093/ajcn/nqz197. Am J Clin Nutr. 2020. PMID: 31907528 Free PMC article. No abstract available.
Abstract
Background: Replacing dietary saturated fatty acids (SFAs) with polyunsaturated fatty acids (PUFA) reduces the plasma low-density lipoprotein (LDL) cholesterol and subsequently the risk of cardiovascular disease. However, beyond changes in LDL cholesterol, we lack a complete understanding of the physiologic alterations that occur when improving dietary fat quality.
Objectives: The aim of this study was to gain knowledge of metabolic alterations paralleling improvements in the fat quality of the diet.
Methods: We recently conducted an 8-wk, double-blind, randomized controlled trial replacing SFAs with PUFAs in healthy subjects with moderate hypercholesterolemia (n = 99). In the present substudy, we performed comprehensive metabolic profiling with multiple platforms (both nuclear magnetic resonance- and mass spectrometry-based technology) (n = 99), and analyzed peripheral blood mononuclear cell gene expression (n = 95) by quantitative real-time polymerase chain reaction.
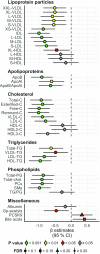
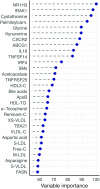
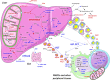
Results: A large number of lipoprotein subclasses, myristoylcarnitine and palmitoylcarnitine, and kynurenine were reduced when SFAs were replaced with PUFAs. In contrast, bile acids, proprotein convertase subtilisin/kexin type 9, acetate, and acetoacetate were increased by the intervention. Some amino acids were also altered by the intervention. The mRNA levels of LXRA and LDLR were increased, in addition to several liver X receptor α target genes and genes involved in inflammation, whereas the mRNA levels of UCP2 and PPARD were decreased in peripheral blood mononuclear cells after replacing SFAs with PUFAs. Partial least squares-discriminant analysis showed that the 30 most important variables that contributed to class separation spanned all classes of biomarkers, and was in accordance with the univariate analysis.
Conclusions: Applying metabolomics in randomized controlled dietary intervention trials has the potential to extend our knowledge of the biological and molecular effects of dietary fat quality. This study was registered at clinicaltrials.gov as NCT01679496.
Keywords: acylcarnitines; cardiovascular risk factors; fatty acids; gene expression; lipoprotein subclasses; metabolic profiling; nutrition; tryptophan.
Copyright © American Society for Nutrition 2019.
Figures



References
-
- Pedersen JI, James PT, Brouwer IA, Clarke R, Elmadfa I, Katan MB, Kris-Etherton PM, Kromhout D, Margetts BM, Mensink RP et al. .. The importance of reducing SFA to limit CHD. Br J Nutr. 2011;106(7):961–3. - PubMed
-
- Hegsted DM, Ausman LM, Johnson JA, Dallal GE. Dietary fat and serum lipids: an evaluation of the experimental data. Am J Clin Nutr. 1993;57(6):875–83. - PubMed
-
- Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb. 1992;12(8):911–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

