Hypoxia Selectively Impairs CAR-T Cells In Vitro
- PMID: 31052261
- PMCID: PMC6562712
- DOI: 10.3390/cancers11050602
Hypoxia Selectively Impairs CAR-T Cells In Vitro
Abstract
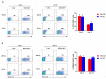
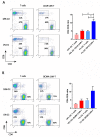
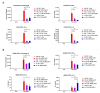
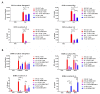
Hypoxia is a major characteristic of the solid tumor microenvironment. To understand how chimeric antigen receptor-T cells (CAR-T cells) function in hypoxic conditions, we characterized CD19-specific and BCMA-specific human CAR-T cells generated in atmospheric (18% oxygen) and hypoxic (1% oxygen) culture for expansion, differentiation status, and CD4:CD8 ratio. CAR-T cells expanded to a much lower extent in 1% oxygen than in 18% oxygen. Hypoxic CAR-T cells also had a less differentiated phenotype and a higher CD4:CD8 ratio than atmospheric CAR-T cells. CAR-T cells were then added to antigen-positive and antigen-negative tumor cell lines at the same or lower oxygen level and characterized for cytotoxicity, cytokine and granzyme B secretion, and PD-1 upregulation. Atmospheric and hypoxic CAR-T cells exhibited comparable cytolytic activity and PD-1 upregulation; however, cytokine production and granzyme B release were greatly decreased in 1% oxygen, even when the CAR-T cells were generated in atmospheric culture. Together, these data show that at solid tumor oxygen levels, CAR-T cells are impaired in expansion, differentiation and cytokine production. These effects may contribute to the inability of CAR-T cells to eradicate solid tumors seen in many patients.
Keywords: BCMA; CAR-T; CD19; hypoxia; immunotherapy; microenvironment; tumor.
Conflict of interest statement
Robert Berahovich, Xianghong Liu, Hua Zhou, Elias Tsadik, Shirley Xu, Vita Golubovskaya and Lijun Wu are employees of Promab Biotechnologies. Other authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

