Sh3bp2 Gain-Of-Function Mutation Ameliorates Lupus Phenotypes in B6.MRL- Faslpr Mice
- PMID: 31052273
- PMCID: PMC6562867
- DOI: 10.3390/cells8050402
Sh3bp2 Gain-Of-Function Mutation Ameliorates Lupus Phenotypes in B6.MRL- Faslpr Mice
Abstract
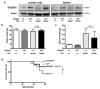
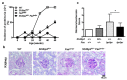
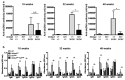
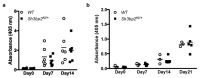
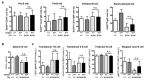
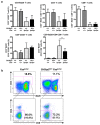
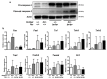
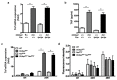
SH3 domain-binding protein 2 (SH3BP2) is an adaptor protein that is predominantly expressed in immune cells, and it regulates intracellular signaling. We had previously reported that a gain-of-function mutation in SH3BP2 exacerbates inflammation and bone loss in murine arthritis models. Here, we explored the involvement of SH3BP2 in a lupus model. Sh3bp2 gain-of-function (P416R knock-in; Sh3bp2KI/+) mice and lupus-prone B6.MRL-Faslpr mice were crossed to yield double-mutant (Sh3bp2KI/+Faslpr/lpr) mice. We monitored survival rates and proteinuria up to 48 weeks of age and assessed renal damage and serum anti-double-stranded DNA antibody levels. Additionally, we analyzed B and T cell subsets in lymphoid tissues by flow cytometry and determined the expression of apoptosis-related molecules in lymph nodes. Sh3bp2 gain-of-function mutation alleviated the poor survival rate, proteinuria, and glomerulosclerosis and significantly reduced serum anti-dsDNA antibody levels in Sh3bp2KI/+Faslpr/lpr mice. Additionally, B220+CD4-CD8- T cell population in lymph nodes was decreased in Sh3bp2KI/+Faslpr/lpr mice, which is possibly associated with the observed increase in cleaved caspase-3 and tumor necrosis factor levels. Sh3bp2 gain-of-function mutation ameliorated clinical and immunological phenotypes in lupus-prone mice. Our findings offer better insight into the unique immunopathological roles of SH3BP2 in autoimmune diseases.
Keywords: Fas; SH3 domain–binding protein 2; anti-dsDNA antibody; dendritic cells; double-negative T cells; lpr mutation; macrophages; murine lupus model; systemic lupus erythematosus; tumor necrosis factor.
Conflict of interest statement
A.N., T.M., K.K., S.T., and Y.M. received scholarship donations from Chugai Pharmaceutical Company. The funder had no role in the design of the study, collection, analyses, or interpretation of data, writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

