Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications
- PMID: 31052294
- PMCID: PMC6562607
- DOI: 10.3390/cells8050403
Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications
Abstract
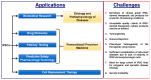
Induced pluripotent stem cell (iPSC)-based disease modelling and the cell replacement therapy approach have proven to be very powerful and instrumental in biomedical research and personalized regenerative medicine as evidenced in the past decade by unraveling novel pathological mechanisms of a multitude of monogenic diseases at the cellular level and the ongoing and emerging clinical trials with iPSC-derived cell products. iPSC-based disease modelling has sparked widespread enthusiasm and has presented an unprecedented opportunity in high throughput drug discovery platforms and safety pharmacology in association with three-dimensional multicellular organoids such as personalized organs-on-chips, gene/base editing, artificial intelligence and high throughput "omics" methodologies. This critical review summarizes the progress made in the past decade with the advent of iPSC discovery in biomedical applications and regenerative medicine with case examples and the current major challenges that need to be addressed to unleash the full potential of iPSCs in clinical settings and pharmacology for more effective and safer regenerative therapy.
Keywords: allogenic cell therapy; autologous cell therapy; cell replacement therapy; clinical trials with stem cells; disease modeling; drug discovery; induced pluripotent stem cells; safety pharmacology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

