Establishment and Expression of Cytokines in a Theileria annulata-Infected Bovine B Cell Line
- PMID: 31052316
- PMCID: PMC6562936
- DOI: 10.3390/genes10050329
Establishment and Expression of Cytokines in a Theileria annulata-Infected Bovine B Cell Line
Abstract
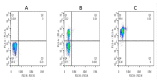
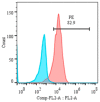
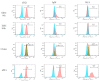
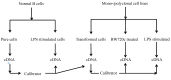
This study aimed to establish a pure single-cell Theileria annulata-infected B cell line for the assessment of cytokine production in transformed and lipopolysaccharide (LPS)-stimulated cells. Several studies have aimed to identify cell surface markers in T. annulata-transformed cells; however, no information on cytokine production in these cells is available. To investigate the potential of the transformed cells to produce cytokines and their potential responses to antigen-stimulation, we purified mature B cells (CD21) from the whole blood of cattle experimentally infected with the T. annulata Kashi strain by magnetic separation. The purity and specificity of the established cell line was assessed by the identification of specific cell surface markers (CD21, IgM, and WC4) by flow cytometry analysis. The transcript levels of the cytokines IL1A, IL1B, IL2, IL4, IL6, IL8, IL10, IL16, LTA, TGFB1, TNFA, IFNA, and IFNB in transformed, buparvaquone (BW720c)-treated cells, and antigen-stimulated cells were analyzed by quantitative polymerase chain reaction (qPCR) using cDNA from these cells. A T. annulata-infected bovine B cell line was successfully established with a purity of ~98.8% (CD21). IL4 and IL12A were significantly (p < 0.01) upregulated in the transformed cells. In BW720c-treated transformed cells, IL12B, TGFB1, and IFNB were significantly (p < 0.01) upregulated. Notably, no significant (p > 0.05) upregulation of cytokines was observed in LPS-stimulated transformed cells. Moreover, IL1A, IL1B, IL8, and IL16 were significantly (p < 0.01) upregulated in LPS-stimulated B cells. Our data signify the potential use of this cell line for cytokine production, observance of immunoglobulins, and production of an attenuated vaccine against tropical theileriosis.
Keywords: B cell line; Theileria annulata; antigenic stimulation; cytokines; transformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Comparative expression profiling of cytokine genes in Theileria annulata-infected and healthy cattle.Trop Anim Health Prod. 2022 Nov 15;54(6):383. doi: 10.1007/s11250-022-03381-7. Trop Anim Health Prod. 2022. PMID: 36380247
-
Macrophage-parasite relationship in theileriosis. Reversible phenotypic and functional dedifferentiation of macrophages infected with Theileria annulata.J Leukoc Biol. 1997 Apr;61(4):459-68. doi: 10.1002/jlb.61.4.459. J Leukoc Biol. 1997. PMID: 9103233
-
Differences between B cell and macrophage transformation by the bovine parasite, Theileria annulata: a clonal approach.J Immunol. 1998 Jul 1;161(1):335-41. J Immunol. 1998. PMID: 9647241
-
Virulence attenuation of Theileria annulata-transformed macrophages.Trends Parasitol. 2025 Apr;41(4):301-316. doi: 10.1016/j.pt.2025.02.007. Epub 2025 Mar 7. Trends Parasitol. 2025. PMID: 40057452 Review.
-
Macrophages behaving badly: infected cells and subversion of immune responses to Theileria annulata.Parasitol Today. 1999 Jan;15(1):10-6. doi: 10.1016/s0169-4758(98)01359-3. Parasitol Today. 1999. PMID: 10234172 Review.
Cited by
-
Effects of Injectable Trace Minerals (ITMs) on Th1/Th2 Cytokine Balance of Newborn Calves with Tropical Theileriosis.Biol Trace Elem Res. 2021 Apr;199(4):1397-1404. doi: 10.1007/s12011-020-02263-z. Epub 2020 Jun 22. Biol Trace Elem Res. 2021. PMID: 32572800
-
Systematic proteomic and small RNA profiling of extracellular vesicles from cattle infected with a naturally occurring buparvaquone-resistant strain of Theileria annulata and from uninfected controls.Parasit Vectors. 2025 Jun 10;18(1):221. doi: 10.1186/s13071-025-06834-8. Parasit Vectors. 2025. PMID: 40495253 Free PMC article.
-
In vitro influence of Theileria annulata on the functions of bovine dendritic cells for stimulation of T lymphocyte proliferation.Parasitology. 2020 Jan;147(1):39-49. doi: 10.1017/S0031182019001227. Epub 2019 Sep 10. Parasitology. 2020. PMID: 31452480 Free PMC article.
-
SNP-based molecular diagnostic platform: rapid single-step identification of Theileria annulata and its buparvaquone-resistant strains.Parasit Vectors. 2025 Jul 1;18(1):247. doi: 10.1186/s13071-025-06884-y. Parasit Vectors. 2025. PMID: 40598565 Free PMC article.
-
Expression of TRAF6 in peripheral blood B cells of patients with myasthenia gravis.BMC Neurol. 2022 Aug 17;22(1):302. doi: 10.1186/s12883-022-02833-9. BMC Neurol. 2022. PMID: 35978310 Free PMC article.
References
-
- Hayashida K., Hara Y., Abe T., Yamasaki C., Toyoda A., Kosuge T., Suzuki Y., Sato Y., Kawashima S., Katayama T. Comparative genome analysis of three eukaryotic parasites with differing abilities to transform leukocytes reveals key mediators of Theileria-induced leukocyte transformation. MBio. 2012;3:e00204-12. doi: 10.1128/mBio.00204-12. - DOI - PMC - PubMed
-
- Wykes M., Pombo A., Jenkins C., MacPherson G.G. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 1998;161:1313–1319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

