The Preparation, Determination of a Flexible Complex Liposome Co-Loaded with Cabazitaxel and β-Elemene, and Animal Pharmacodynamics on Paclitaxel-Resistant Lung Adenocarcinoma
- PMID: 31052317
- PMCID: PMC6539285
- DOI: 10.3390/molecules24091697
The Preparation, Determination of a Flexible Complex Liposome Co-Loaded with Cabazitaxel and β-Elemene, and Animal Pharmacodynamics on Paclitaxel-Resistant Lung Adenocarcinoma
Abstract
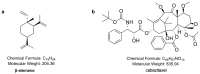
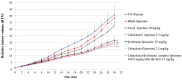
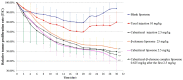
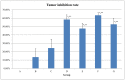
Paclitaxel is highly effective at killing many malignant tumors; however, the development of drug resistance is common in clinical applications. The issue of overcoming paclitaxel resistance is a difficult challenge at present. In this study, we developed nano drugs to treat paclitaxel-resistant lung adenocarcinoma. We selected cabazitaxel and β-elemene, which have fewer issues with drug resistance, and successfully prepared cabazitaxel liposome, β-elemene liposome and cabazitaxel-β-elemene complex liposome with good flexibility. The encapsulation efficiencies of cabazitaxel and β-elemene in these liposomes were detected by precipitation microfiltration and microfiltration centrifugation methods, respectively. Their encapsulation efficiencies were all above 95%. The release rates were detected by a dialysis method. The release profiles of cabazitaxel and β-elemene in these liposomes conformed to the Weibull equation. The release of cabazitaxel and β-elemene in the complex liposome were almost synchronous. The pharmacodynamics study showed that cabazitaxel flexible liposome and β-elemene flexible liposome were relatively good at overcoming paclitaxel resistance on paclitaxel-resistant lung adenocarcinoma. As the flexible complex liposome, the dosage of cabazitaxel could be reduced to 25% that of the cabazitaxel injection while retaining a similar therapeutic effect. It showed that β-elemene can replace some of the cabazitaxel, allowing the dosage of cabazitaxel to be reduced, thereby reducing the drug toxicity.
Keywords: cabazitaxel; liposome; lung adenocarcinoma; paclitaxel resistance; β-elemene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Preparation, characterization, pharmacokinetics and anticancer effects of PEGylated β-elemene liposomes.Cancer Biol Med. 2020 Feb 15;17(1):60-75. doi: 10.20892/j.issn.2095-3941.2019.0156. Cancer Biol Med. 2020. PMID: 32296587 Free PMC article.
-
In vitro combination characterization of the new anticancer plant drug beta-elemene with taxanes against human lung carcinoma.Int J Oncol. 2007 Aug;31(2):241-52. Int J Oncol. 2007. PMID: 17611679
-
Anti-EGFR immunoliposomes for cabazitaxel delivery: From formulation development to in vivo evaluation in prostate cancer xenograft model.Int J Pharm. 2024 Aug 15;661:124439. doi: 10.1016/j.ijpharm.2024.124439. Epub 2024 Jul 6. Int J Pharm. 2024. PMID: 38972520
-
Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy.Int J Nanomedicine. 2018 Oct 10;13:6279-6296. doi: 10.2147/IJN.S174527. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30349250 Free PMC article. Review.
-
Preclinical profile of cabazitaxel.Drug Des Devel Ther. 2014 Oct 13;8:1851-67. doi: 10.2147/DDDT.S64940. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25378905 Free PMC article. Review.
Cited by
-
β-elemene down-regulates HIF-lα, VEGF and iNOS in human retinal pigment epithelial cells under high glucose conditions.Int J Ophthalmol. 2020 Dec 18;13(12):1887-1894. doi: 10.18240/ijo.2020.12.07. eCollection 2020. Int J Ophthalmol. 2020. PMID: 33344186 Free PMC article.
-
Inhibiting the NF-κB pathway enhances the antitumor effect of cabazitaxel by downregulating Bcl-2 in pancreatic cancer.Int J Oncol. 2020 Jul;57(1):161-170. doi: 10.3892/ijo.2020.5053. Epub 2020 Apr 27. Int J Oncol. 2020. PMID: 32377719 Free PMC article.
-
Harnessing the Power of Traditional Chinese Medicine in Cancer Treatment: The Role of Nanocarriers.Int J Nanomedicine. 2025 Mar 13;20:3147-3174. doi: 10.2147/IJN.S502104. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40103746 Free PMC article. Review.
-
An ATF24 peptide-functionalized β-elemene-nanostructured lipid carrier combined with cisplatin for bladder cancer treatment.Cancer Biol Med. 2020 Aug 15;17(3):676-692. doi: 10.20892/j.issn.2095-3941.2020.0454. Cancer Biol Med. 2020. PMID: 32944399 Free PMC article.
-
A surfactant-stripped cabazitaxel micelle formulation optimized with accelerated storage stability.Pharm Dev Technol. 2020 Dec;25(10):1281-1288. doi: 10.1080/10837450.2020.1818780. Epub 2020 Sep 16. Pharm Dev Technol. 2020. PMID: 32892678 Free PMC article.
References
-
- Kenicer J., Spears M., Lyttle N., Taylor K.J., Liao L., Cunningham C.A., Lambros M., MacKay A., Yao C., Reis-Filho J., et al. Molecular characterisation of isogenic taxane resistant cell lines identify novel drivers of drug resistance. BMC Cancer. 2014;14:762. doi: 10.1186/1471-2407-14-762. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

