A Functional Wound Dressing as a Potential Treatment for Cutaneous Leishmaniasis
- PMID: 31052360
- PMCID: PMC6571773
- DOI: 10.3390/pharmaceutics11050200
A Functional Wound Dressing as a Potential Treatment for Cutaneous Leishmaniasis
Abstract
Cutaneous leishmaniasis (CL) is a parasitic disease characterized by progressive skin sores. Currently, treatments for CL are limited to parenteral administration of the drug, which presents severe adverse effects and low cure rates. Therefore, this study aimed to develop poly(vinyl-alcohol) (PVA) hydrogels containing Amphotericin B (AmB) intended for topical treatment of CL. Hydrogels were evaluated in vitro for their potential to eliminate promastigote forms of Leishmania spp., to prevent secondary infections, to maintain appropriate healing conditions, and to offer suitable biocompatibility. AmB was incorporated into the system in its non-crystalline state, allowing it to swell more and faster than the system without the drug. Furthermore, the AmB release profile showed a continuous and controlled behavior following Higuchi´s kinetic model. AmB-loaded-PVA-hydrogels (PVA-AmB) also showed efficient antifungal and leishmanicidal activity, no cytotoxic potential for VERO cells, microbial impermeability and water vapor permeability compatible with the healthy skin's physiological needs. Indeed, these results revealed the potential of PVA-AmB to prevent secondary infections and to maintain a favorable environment for the healing process. Hence, these results suggest that PVA-AmB could be a suitable and efficient new therapeutic approach for the topical treatment of CL.
Keywords: Amphotericin B; controlled release; cutaneous leishmaniasis; hydrogel; wound dressing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

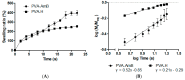



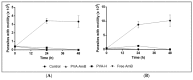

References
-
- Alvar J., Arana B.I. Drug Discovery for Leishmaniasis. The Royal Society of Chemistry; London, UK: 2018. Appraisal of Leishmaniasis Chemotherapy, Current Status and Pipeline StrategiesChapter 1 Leishmaniasis, Impact and Therapeutic Needs; pp. 1–13.
-
- WHO . Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Tropical Diseases. WHO Library; Paris, France: 2013. pp. 67–71.
-
- WHO . Leishmaniasis—Fact sheet N°375. WHO; Geneva, Switzerland: 2014.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

