Crystallization Tendency of Pharmaceutical Glasses: Relevance to Compound Properties, Impact of Formulation Process, and Implications for Design of Amorphous Solid Dispersions
- PMID: 31052392
- PMCID: PMC6572324
- DOI: 10.3390/pharmaceutics11050202
Crystallization Tendency of Pharmaceutical Glasses: Relevance to Compound Properties, Impact of Formulation Process, and Implications for Design of Amorphous Solid Dispersions
Abstract
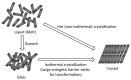
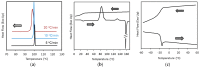
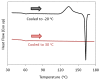
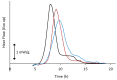
Amorphous solid dispersions (ASDs) are important formulation strategies for improving the dissolution process and oral bioavailability of poorly soluble drugs. Physical stability of a candidate drug must be clearly understood to design ASDs with superior properties. The crystallization tendency of small organics is frequently estimated by applying rapid cooling or a cooling/reheating cycle to their melt using differential scanning calorimetry. The crystallization tendency determined in this way does not directly correlate with the physical stability during isothermal storage, which is of great interest to pharmaceutical researchers. Nevertheless, it provides important insights into strategy for the formulation design and the crystallization mechanism of the drug molecules. The initiation time for isothermal crystallization can be explained using the ratio of the glass transition and storage temperatures (Tg/T). Although some formulation processes such as milling and compaction can enhance nucleation, the Tg/T ratio still works for roughly predicting the crystallization behavior. Thus, design of accelerated physical stability test may be possible for ASDs. The crystallization tendency during the formulation process and the supersaturation ability of ASDs may also be related to the crystallization tendency determined by thermal analysis. In this review, the assessment of the crystallization tendency of pharmaceutical glasses and its relevance to developmental studies of ASDs are discussed.
Keywords: accelerated stability test; crystallization; crystallization tendency; milling; nucleation; pharmaceutical glass.
Conflict of interest statement
The author declares no conflicts of interest.
Figures





Similar articles
-
Supersaturation and crystallization: non-equilibrium dynamics of amorphous solid dispersions for oral drug delivery.Expert Opin Drug Deliv. 2017 Jun;14(6):735-743. doi: 10.1080/17425247.2017.1230099. Epub 2016 Sep 9. Expert Opin Drug Deliv. 2017. PMID: 27598556 Review.
-
Relationship between crystallization tendencies during cooling from melt and isothermal storage: toward a general understanding of physical stability of pharmaceutical glasses.Mol Pharm. 2014 Jun 2;11(6):1835-43. doi: 10.1021/mp400679m. Epub 2014 Apr 25. Mol Pharm. 2014. PMID: 24731254
-
Hydroxypropyl cellulose stabilizes amorphous solid dispersions of the poorly water soluble drug felodipine.Carbohydr Polym. 2014 Nov 4;112:512-9. doi: 10.1016/j.carbpol.2014.06.039. Epub 2014 Jun 21. Carbohydr Polym. 2014. PMID: 25129775
-
Understanding the glass-forming ability of active pharmaceutical ingredients for designing supersaturating dosage forms.J Pharm Sci. 2012 Sep;101(9):3239-48. doi: 10.1002/jps.23166. Epub 2012 Apr 24. J Pharm Sci. 2012. PMID: 22531946
-
Classification of solid dispersions: correlation to (i) stability and solubility (ii) preparation and characterization techniques.Drug Dev Ind Pharm. 2015;41(9):1401-15. doi: 10.3109/03639045.2015.1018274. Epub 2015 May 20. Drug Dev Ind Pharm. 2015. PMID: 25853292 Review.
Cited by
-
Opportunities for Successful Stabilization of Poor Glass-Forming Drugs: A Stability-Based Comparison of Mesoporous Silica Versus Hot Melt Extrusion Technologies.Pharmaceutics. 2019 Nov 4;11(11):577. doi: 10.3390/pharmaceutics11110577. Pharmaceutics. 2019. PMID: 31689980 Free PMC article.
-
Fragility and Tendency to Crystallization for Structurally Related Compounds.Int J Mol Sci. 2024 Mar 11;25(6):3200. doi: 10.3390/ijms25063200. Int J Mol Sci. 2024. PMID: 38542174 Free PMC article.
-
Study of Thermal Properties, Molecular Dynamics, and Physical Stability of Etoricoxib Mixtures with Octaacetylmaltose near the Glass Transition.Int J Mol Sci. 2022 Aug 29;23(17):9794. doi: 10.3390/ijms23179794. Int J Mol Sci. 2022. PMID: 36077212 Free PMC article.
-
Influence of Pore Size of Mesoporous Silica on Physical Stability of Overloaded Celecoxib Glass.Mol Pharm. 2025 May 5;22(5):2556-2567. doi: 10.1021/acs.molpharmaceut.4c01482. Epub 2025 Apr 4. Mol Pharm. 2025. PMID: 40183771 Free PMC article.
-
A Comparative Study of the Pharmaceutical Properties between Amorphous Drugs Loaded-Mesoporous Silica and Pure Amorphous Drugs Prepared by Solvent Evaporation.Pharmaceuticals (Basel). 2022 Jun 9;15(6):730. doi: 10.3390/ph15060730. Pharmaceuticals (Basel). 2022. PMID: 35745649 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

