Entropic Forces Drive Cellular Contact Guidance
- PMID: 31053262
- PMCID: PMC6531843
- DOI: 10.1016/j.bpj.2019.04.003
Entropic Forces Drive Cellular Contact Guidance
Abstract
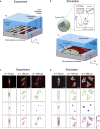
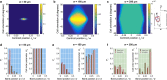
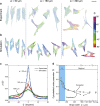
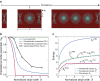
Contact guidance-the widely known phenomenon of cell alignment induced by anisotropic environmental features-is an essential step in the organization of adherent cells, but the mechanisms by which cells achieve this orientational ordering remain unclear. Here, we seeded myofibroblasts on substrates micropatterned with stripes of fibronectin and observed that contact guidance emerges at stripe widths much greater than the cell size. To understand the origins of this surprising observation, we combined morphometric analysis of cells and their subcellular components with a, to our knowledge, novel statistical framework for modeling nonthermal fluctuations of living cells. This modeling framework is shown to predict not only the trends but also the statistical variability of a wide range of biological observables, including cell (and nucleus) shapes, sizes, and orientations, as well as stress-fiber arrangements within the cells with remarkable fidelity with a single set of cell parameters. By comparing observations and theory, we identified two regimes of contact guidance: 1) guidance on stripe widths smaller than the cell size (w ≤ 160 μm), which is accompanied by biochemical changes within the cells, including increasing stress-fiber polarization and cell elongation; and 2) entropic guidance on larger stripe widths, which is governed by fluctuations in the cell morphology. Overall, our findings suggest an entropy-mediated mechanism for contact guidance associated with the tendency of cells to maximize their morphological entropy through shape fluctuations.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Chien K.R., Domian I.J., Parker K.K. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322:1494–1497. - PubMed
-
- Provenzano P.P., Vanderby R., Jr. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

