Generation of transgenic zebrafish with 2 populations of RFP- and GFP-labeled thrombocytes: analysis of their lipids
- PMID: 31053568
- PMCID: PMC6517667
- DOI: 10.1182/bloodadvances.2018023960
Generation of transgenic zebrafish with 2 populations of RFP- and GFP-labeled thrombocytes: analysis of their lipids
Abstract
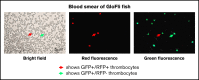
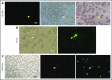
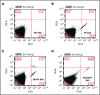
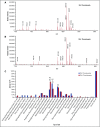
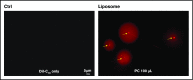
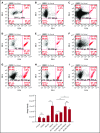
Zebrafish thrombocytes are similar to mammalian platelets. Mammals have young platelets (also called reticulated platelets) and mature platelets. Likewise, zebrafish have 2 populations of thrombocytes; one is DiI-C18 (DiI)+ (DP), and the other is DiI- (DN). However, the mechanism of selective thrombocyte labeling by DiI is unknown. Furthermore, there is no transgenic zebrafish line where DP and DN thrombocytes are differentially labeled with fluorescent proteins. In this study, we found that Glo fish, in which the myosin light chain 2 promoter drives the rfp gene, have a population of thrombocytes that are red fluorescent protein (RFP) labeled. We also generated transgenic GloFli fish in which DP and DN thrombocytes are labeled with RFP and green fluorescent protein (GFP), respectively. Single-cell lipid analysis showed a twofold increase in phosphatidylethanolamine (PE) and a twofold decrease in phosphatidylcholine (PC) in RFP+ thrombocytes compared with GFP+ thrombocytes, suggesting that lipid composition may be important for DiI differential labeling. Therefore, we tested liposomes prepared with different ratios of PC and PE and observed that liposomes prepared with higher amounts of PE favor DiI labeling, whereas the PC concentration had a modest effect. In liposomes prepared using only PE or PC, increased concentrations of PE resulted in increased DiI binding. These results suggest that because RFP+ thrombocytes have higher PE concentrations, DiI may bind to them efficiently, thus explaining the selective labeling of thrombocytes by DiI. This work also provides GloFli fish that should be useful in understanding the mechanism of thrombocyte maturation.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
References
-
- Jagadeeswaran P, Sheehan JP, Craig FE, Troyer D. Identification and characterization of zebrafish thrombocytes. Br J Haematol. 1999;107(4):731-738. - PubMed
-
- Gregory M, Jagadeeswaran P. Selective labeling of zebrafish thrombocytes: quantitation of thrombocyte function and detection during development. Blood Cells Mol Dis. 2002;28(3):418-427. - PubMed
-
- Klymchenko AS, Kreder R. Fluorescent probes for lipid rafts: from model membranes to living cells. Chem Biol. 2014;21(1):97-113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

