Synchronicity: The Role of Midbrain Dopamine in Whole-Brain Coordination
- PMID: 31053604
- PMCID: PMC6500793
- DOI: 10.1523/ENEURO.0345-18.2019
Synchronicity: The Role of Midbrain Dopamine in Whole-Brain Coordination
Abstract
Midbrain dopamine seems to play an outsized role in motivated behavior and learning. Widely associated with mediating reward-related behavior, decision making, and learning, dopamine continues to generate controversies in the field. While many studies and theories focus on what dopamine cells encode, the question of how the midbrain derives the information it encodes is poorly understood and comparatively less addressed. Recent anatomical studies suggest greater diversity and complexity of afferent inputs than previously appreciated, requiring rethinking of prior models. Here, we elaborate a hypothesis that construes midbrain dopamine as implementing a Bayesian selector in which individual dopamine cells sample afferent activity across distributed brain substrates, comprising evidence to be evaluated on the extent to which stimuli in the on-going sensorimotor stream organizes distributed, parallel processing, reflecting implicit value. To effectively generate a temporally resolved phasic signal, a population of dopamine cells must exhibit synchronous activity. We argue that synchronous activity across a population of dopamine cells signals consensus across distributed afferent substrates, invigorating responding to recognized opportunities and facilitating further learning. In framing our hypothesis, we shift from the question of how value is computed to the broader question of how the brain achieves coordination across distributed, parallel processing. We posit the midbrain is part of an "axis of agency" in which the prefrontal cortex (PFC), basal ganglia (BGS), and midbrain form an axis mediating control, coordination, and consensus, respectively.
Keywords: coherence; dopamine; phasic dopamine; striatum; synchronous dopamine activity.
Copyright © 2019 Beeler and Dreyer.
Figures
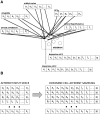
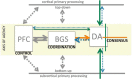
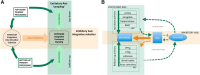


References
-
- Agnati LF, Zoli M, Strömberg I, Fuxe K (1995) Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69:711–726. - PubMed
-
- Alexander GE (1994) Basal ganglia-thalamocortical circuits: their role in control of movements. J Clin Neurophysiol 11:420–431. - PubMed
-
- Barto AG (1995) Adaptive critic and the basal ganglia In: Models of Information Processing in the Basal Ganglia, pp 215–232. Cambridge, MA: MIT Press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
